Modification Strategy of D-leucine Residue Addition on a Novel Peptide from Odorrana schmackeri, with Enhanced Bioactivity and In Vivo Efficacy
Abstract
:1. Introduction
2. Results
2.1. “Shotgun” Cloning of B1OS from Odorrana schmackeri Skin Secretion-Derived cDNA Library
2.2. Conformational Analysis of B1OS, B1OS-L and B1OS-D-L
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of B1OS, B1OS-Lm and D-B1OS-L
2.4. Prevention and Eradication of Biofilm by B1OS, B1OS-L and B1OS-D-L
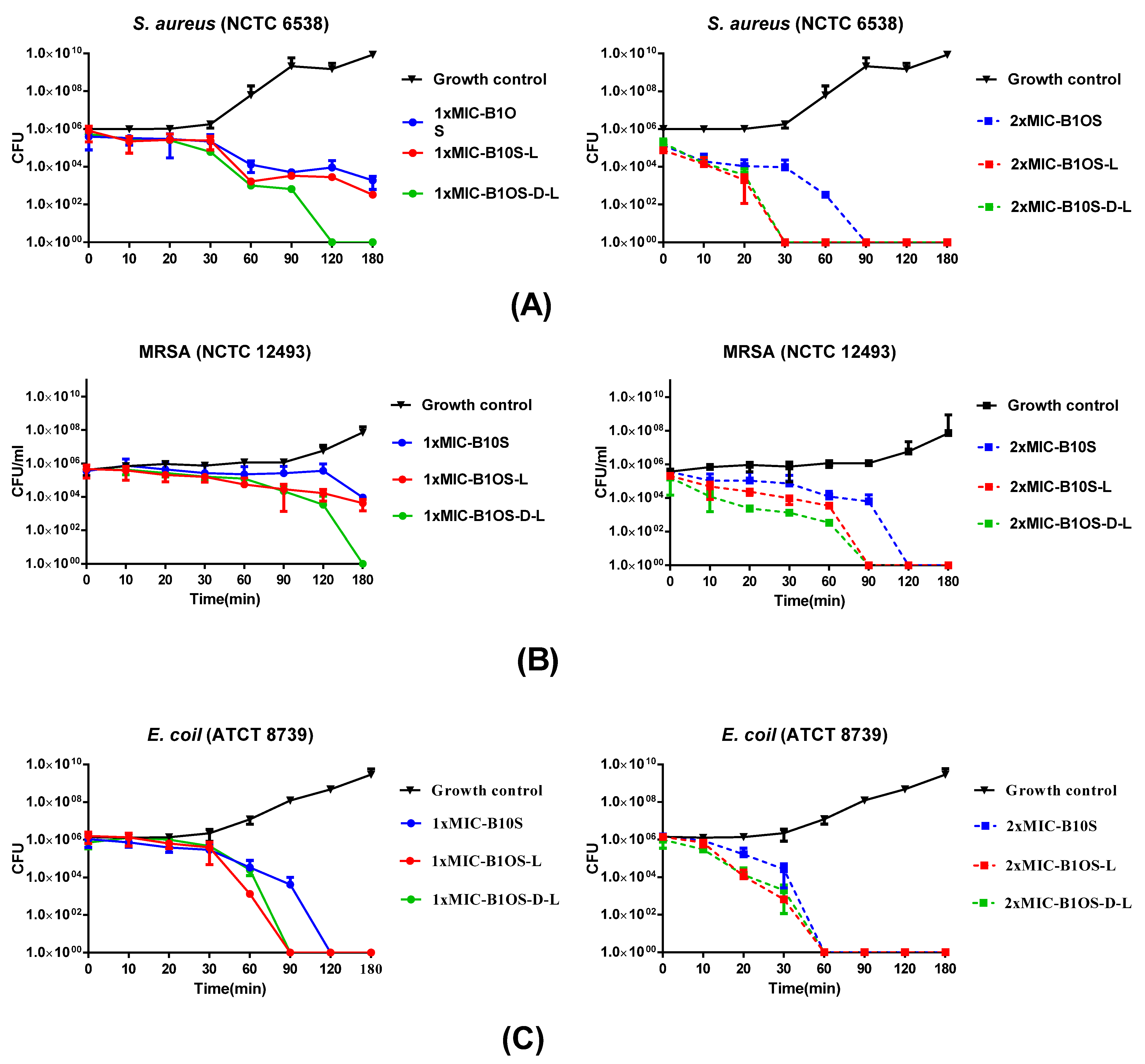
2.5. Time-Killing Kinetics of Peptides against S. aureus, MRSA and E. coli
2.6. Membrane Permeability of MRSA by B1OS, B1OS-L and B1OS-D-L
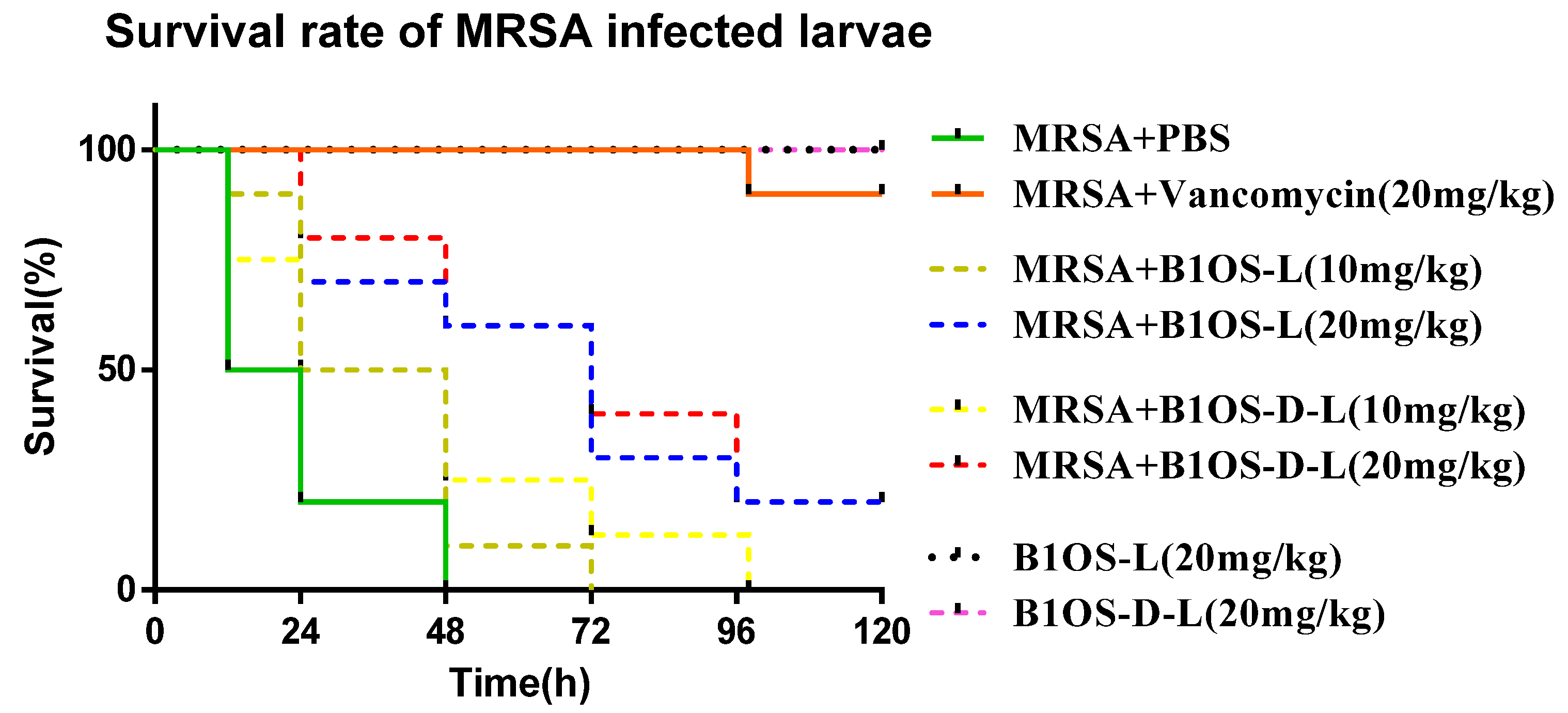
2.7. Treatment of MRSA-Infected Waxworms with B1OS-L and B1OS-D-L
2.8. Anticancer Proliferative Activity of B1OS, B1OS-L and B1OS-D-L
2.9. Cytotoxicity of B1OS, B1OS-L and B1OS-D-L
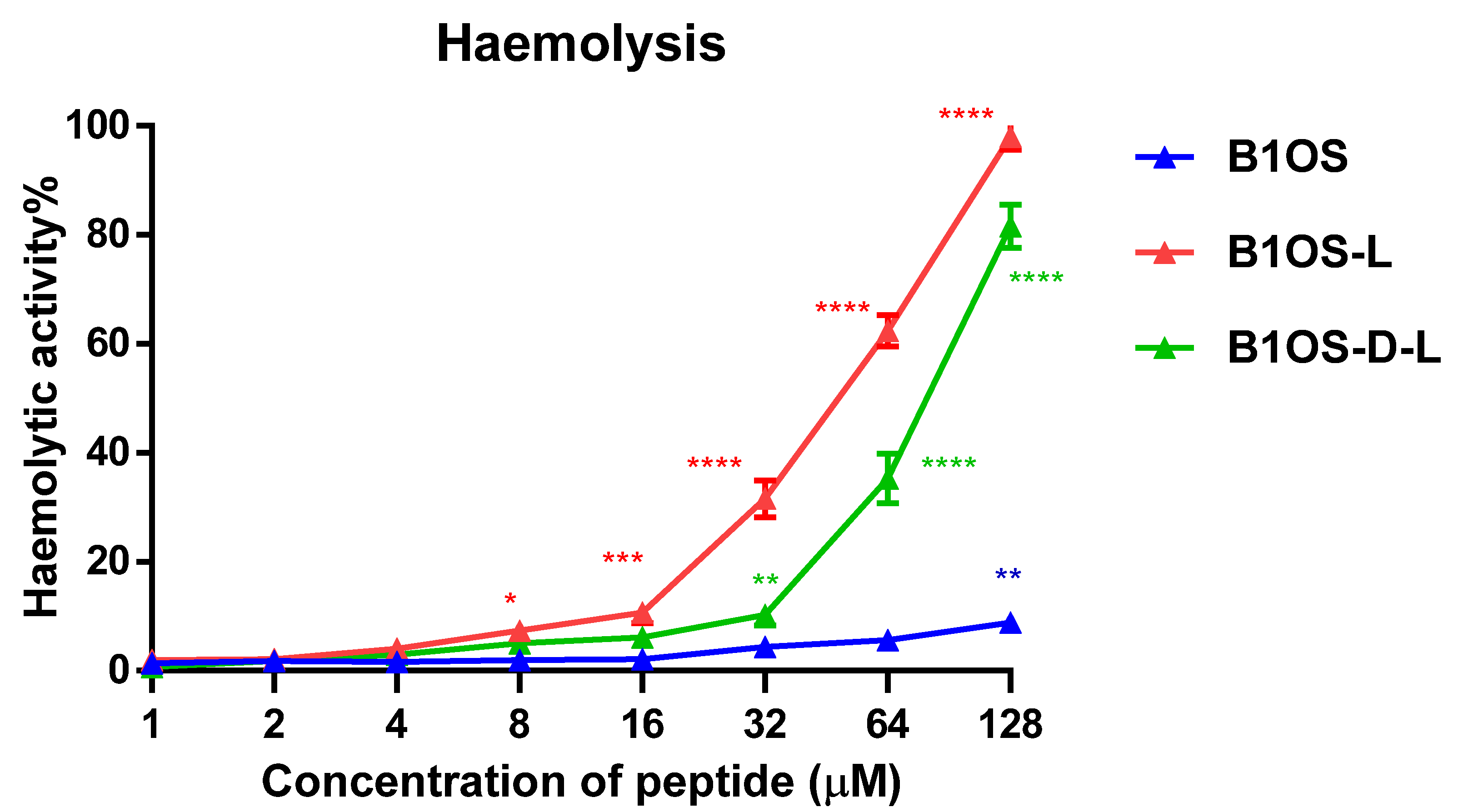
2.10. Haemolytic Activity of B1OS, B1OS-L and B1OS-D-L
3. Discussion
4. Materials and Methods
4.1. Acquisition of Odorrana schmackeri Skin Secretion
4.2. “Shotgun” Cloning of B1OS from Odorrana schmackeri Skin Secretion-Derived cDNA Library
4.3. Synthesis and Purification of B1OS, B10S-L and B1OS-D-L
4.4. Prediction of Peptide Structure
4.5. CD Spectra
4.6. MIC and MBC Assays
4.7. MBIC and MBEC Assays
4.8. Time-Killing Assays
4.9. Bacterial Membrane Permeability Assays
4.10. Efficacy Evaluation of B1OS-L against MRSA in Larvae
4.11. Anticancer Proliferative MTT Assays
4.12. Trypan Blue Exclusion Assay
4.13. Haemolysis Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Munita, J.M.; Bayer, A.S.; Arias, C.A. Evolving resistance among Gram-positive pathogens. Clin. Infect. Dis. 2015, 61 (Suppl. 2), 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenberger, E.; Thaden, J.T. Epidemiology and Mechanisms of Resistance of Extensively Drug Resistant Gram-Negative Bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Karim-Kos, H.E.; Coebergh, J.W.; Byrnes, G.; Antilla, A.; Ferlay, J.; Renehan, A.G.; Forman, D.; Soerjomataram, I. Recent Rends in Incidence of Five Common Cancers in 26 European Countries Since 1988: Analysis of the European Cancer Observatory. Eur. J. Cancer 2015, 51, 1164–1187. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V. The spectrum of pulmonary infections in cancer patients. Curr. Opin. Oncol. 2001, 13, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Mechkarska, M.; Lukic, M.; Flatt, P. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 2014, 57, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Zasloff, M. Peptides from Frog Skin. Annu. Rev. Biochem. 1990, 59, 395–414. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Morikawa, N.; Hagiwara, K.; Nakajima, T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992, 189, 184–190. [Google Scholar] [CrossRef]
- Zohrab, F.; Askarian, S.; Jalili, A.; Oskuee, R.K. Biological Properties, Current Applications and Potential Therapeautic Applications of Brevinin Peptide Superfamily. Int. J. Pept. Res. Ther. 2018, 25, 39–48. [Google Scholar] [CrossRef]
- Eckert, R. Road to clinical efficacy: Challenges and novel strategies for antimicrobial peptide development. Futur. Microbiol. 2011, 6, 635–651. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Hu, Y.; Li, F.; Liu, L.; Zheng, H.; Meng, H.; Yang, S.; Yang, X.; Liu, J. Novel antimicrobial peptides isolated from the skin secretions of Hainan odorous frog, Odorrana hainanensis. Peptides 2012, 35, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Zhou, M.; Chen, W.; Chen, T.; Walker, B.; Shaw, C. Novel brevinins from Chinese piebald odorous frog (Huia schmackeri) skin deduced from cloned biosynthetic precursors. Peptides 2008, 29, 1456–1460. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2014, 12, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. I-TASSER Server For Protein 3D Structure Prediction. BMC Bioinform. 2008, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zai, Y.; Ying, Y.; Ye, Z.; Zhou, M.; Ma, C.; Shi, Z.; Chen, X.; Xi, X.; Chen, T.; Wang, L. Broad-Spectrum Antimicrobial Activity and Improved Stability of a D-Amino Acid Enantiomer of DMPC-10A, the Designed Derivative of Dermaseptin Truncates. Antibiotics 2020, 9, 627. [Google Scholar] [CrossRef]
- Genovese, C.; D’Angeli, F.; Bellia, F.; Distefano, A.; Spampinato, M.; Attanasio, F.; Nicolosi, D.; Di Salvatore, V.; Tempera, G.; Lo Furno, D.; et al. In Vitro Antibacterial, Anti-Adhesive and Anti-Biofilm Activities of Krameria lappacea (Dombey) Burdet & B.B. Simpson Root Extract against Methicillin-Resistant Staphylococcus aureus Strains. Antibiotics 2021, 10, 428. [Google Scholar] [CrossRef]
- Rohl, C.A.; Fiori, W.; Baldwin, R.L. Alanine is helix-stabilizing in both template-nucleated and standard peptide helices. Proc. Natl. Acad. Sci. USA 1999, 96, 3682–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuerkova, A.; Kabelka, I.; Králová, T.; Sukeník, L.; Pokorná, Š.; Hof, M.; Vácha, R. Effect of Helical Kink in Antimicrobial Peptides on Membrane Pore Formation. eLife 2020, 9, e47946. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-Y.; Hong, S.-Y.; Lee, K.-H. Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esculenta. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1998, 1387, 239–248. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Song, J.-W.; Gong, F.; Li, S.-B.; Chang, H.-Y.; Xie, H.-M.; Gao, H.-W.; Tan, Y.-X.; Ji, S.-P. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef] [Green Version]
- Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Somsri, S.; Aunpad, R. A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, Y.; Wang, T.; Chen, X.; Zhou, M.; Ma, C.; Xi, X.; Zhang, Y.; Chen, T.; Shaw, C.; et al. Brevinin-1GHd: A novel Hylarana guentheri skin secretion-derived Brevinin-1 type peptide with antimicrobial and anticancer therapeutic potential. Biosci. Rep. 2020, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Dermaseptin-PH: A Novel Peptide with Antimicrobial and Anticancer Activities from the Skin Secretion of the South American Orange-Legged Leaf Frog, Pithecopus (Phyllomedusa) hypochondrialis. Molecules 2017, 22, 1805. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Ma, C.; Zhang, Y.; Xi, X.; Wang, L.; Zhou, M.; Burrows, J.F.; Chen, T. A novel antimicrobial peptide, Ranatuerin-2PLx, showing therapeutic potential in inhibiting proliferation of cancer cells. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roccatano, D.; Colombo, G.; Fioroni, M.; Mark, A. Mechanism by which 2,2,2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: A molecular dynamics study. Proc. Natl. Acad. Sci. USA 2002, 99, 12179–12184. [Google Scholar] [CrossRef] [Green Version]
- Reiersen, H.; Rees, A.R. Trifluoroethanol may form a solvent matrix for assisted hydrophobic interactions between peptide side chains. Protein Eng. 2000, 13, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, L.S.; Pande, J.; Shekhtman, A. Helical Structure of Recombinant Melittin. J. Phys. Chem. B 2018, 123, 356–368. [Google Scholar] [CrossRef]
- Lee, T.-H.; Hall, K.N.; Aguilar, M.-I. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2015, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-W.; Wei, S.-Y.; Wang, S.-H.; Wei, H.-M.; Wang, Y.-J.; Wang, C.-F.; Chen, C.; Liao, Y.-D. Hydrophobic residues are critical for the helix-forming, hemolytic and bactericidal activities of amphipathic antimicrobial peptide TP4. PLoS ONE 2017, 12, e0186442. [Google Scholar] [CrossRef] [Green Version]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta (BBA) Biomembr. 1999, 1462, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Pouny, Y.; Rapaport, D.; Mor, A.; Nicolas, P.; Shai, Y. Interaction of Antimicrobial Dermaseptin and its Fluorescently Labeled Analogues with Phospholipid Membranes. Biochemistry 1992, 31, 12416–12423. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.F., Jr.; Vanhoye, D.; Nicolas, P. Roles of Diversifying Selection and Coordinated Evolution in the Evolution of Amphibian Antimicrobial Peptides. Mol. Biol. Evol. 2002, 19, 858–864. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, P.; Vanhoye, D.; Amiche, M. Molecular strategies in biological evolution of antimicrobial peptides. Peptides 2003, 24, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.A. Molecular evolution of animal antimicrobial peptides: Widespread moderate positive selection. J. Evol. Biol. 2005, 18, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.; Kolodziejek, J.; Nowotny, N. Antimicrobial peptides from ranid frogs: Taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2003, 1696, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Sharma, V. D-Amino Acids in Antimicrobial Peptides: A Potential Approach to Treat and Combat Antimicrobial Resistance. Can. J. Microbiol. 2021, 67, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, H.M.; Feix, J.B. Effects of D-Lysine Substitutions on the Activity and Selectivity of Antimicrobial Peptide CM15. Polymers 2011, 3, 2088–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; He, L.; Li, G.; Zhai, N.; Jiang, H.; Chen, Y. Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.E.; Lee, K.H. Synthesis of novel unnatural amino acid as a building block and its incorporation into an antimicrobial peptide. Bioorganic Med. Chem. 1999, 7, 2985–2990. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Lev, N.; Shai, Y. Effect of the Hydrophobicity to Net Positive Charge Ratio on Antibacterial and Anti-Endotoxin Activities of Structurally Similar Antimicrobial Peptides. Biochemistry 2010, 49, 853–861. [Google Scholar] [CrossRef]
- Fjell, C.; Hiss, J.A.; Hancock, R.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.; Macdonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Zhang, J.; Hu, X.; Li, Z.; Fa, K.; Liu, H.; Waigh, T.A.; McBain, A.; Lu, J.R. Hydrophobic Control of the Bioactivity and Cytotoxicity of de Novo-Designed Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2019, 11, 34609–34620. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of Peptide Hydrophobicity in the Mechanism of Action of α-Helical Antimicrobial Peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Auer, G.K.; Weibel, D.B. Bacterial Cell Mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2016, 96, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the Bacterial Cell Envelope by Antimicrobial Peptides Gramicidin S and PGLa as Revealed by Transmission and Scanning Electron Microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Pan, J.; Wu, Y.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Chen, T. PSN-PC: A Novel Antimicrobial and Anti-Biofilm Peptide from the Skin Secretion of Phyllomedusa-camba with Cytotoxicity on Human Lung Cancer Cell. Molecules 2017, 22, 1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N.; Leprince, J.; Vaudry, H.; Coquet, L.; Jouenne, T.; Iwamuro, S. Cytolytic peptides belonging to the brevinin-1 and brevinin-2 families isolated from the skin of the Japanese brown frog, Rana dybowskii. Toxicon 2007, 50, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Gao, Y.; Wang, Y.; Xia, Q.; Zhong, R.; Ma, C.; Zhou, M.; Xi, X.; Shaw, C.; et al. Enhanced Antimicrobial Activity of N-Terminal Derivatives of a Novel Brevinin-1 Peptide from The Skin Secretion of Odorrana schmackeri. Toxins 2020, 12, 484. [Google Scholar] [CrossRef]
- Pei, X.; Gong, Z.; Wu, Q.; Chen, X.; Wang, L.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Characterisation of a novel peptide, Brevinin-1H, from the skin secretion of Amolops hainanensis and rational design of several analogues. Chem. Biol. Drug Des. 2020, 97, 273–282. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, Y.; Ye, Z.; Chen, X.; Ma, C.; Zhou, M.; Xi, X.; Burrows, J.F.; Chen, T.; Wang, L. Modification and Targeted Design of N-Terminal Truncates Derived from Brevinin with Improved Therapeutic Efficacy. Biology 2020, 9, 209. [Google Scholar] [CrossRef]
- Kavanagh, K.; Reeves, E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2013, 69, 121–132. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Desbois, A.; Coote, P.J. Wax moth larva (Galleria mellonella): An in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
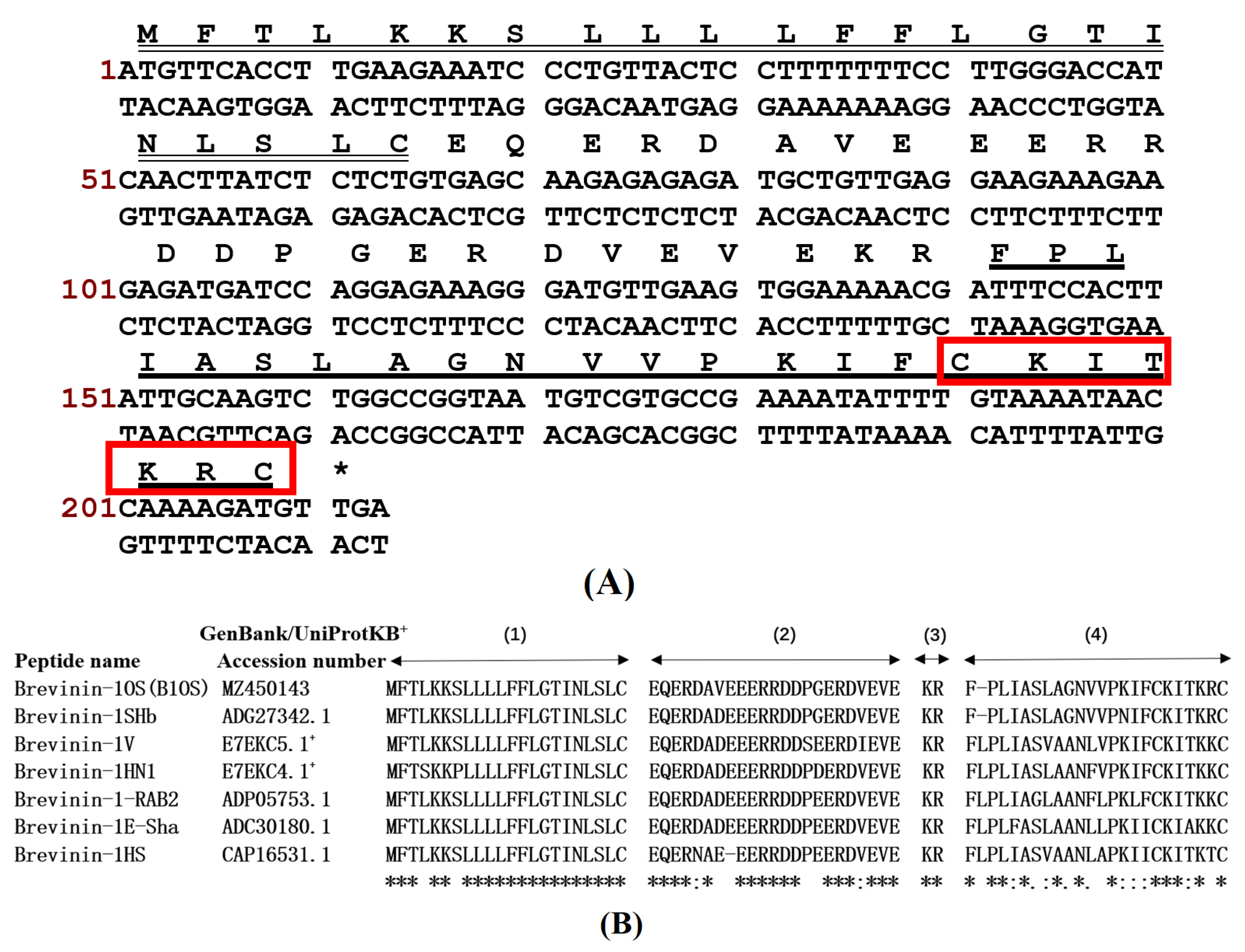
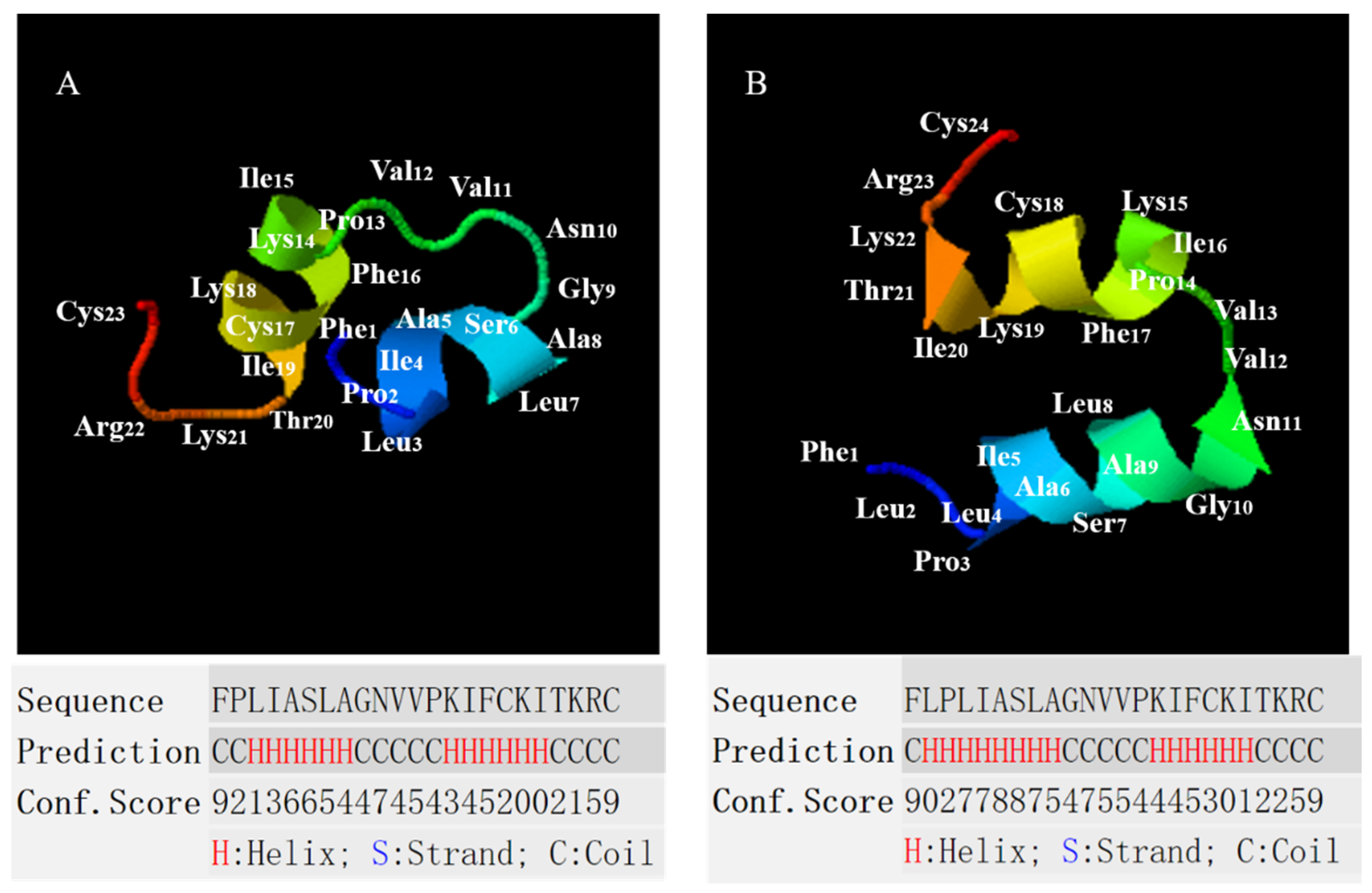




| Parameters. | B1OS | B1OS-L | B1OS-D-L |
|---|---|---|---|
| Hydrophobicity (H) | 0.678 | 0.721 | 0.721 |
| Hydrophobic moment (µH) | 0.333 | 0.291 | 0.291 |
| Net charge (z) | +4 | +4 | +4 |
| Peptide sequence | FPLIASLAGNVVPKIFCKITKRC | FLPLIASLAGNVVPKIFCKITKRC | FLPLIASLAGNVVPKIFCKITKRC |
| Microorganisms | MICs/MBCs (µM) | MICs (μM) | |||
|---|---|---|---|---|---|
| B1OS | B1OS-L | B1OS-D-L | Norfloxacin a | ||
| Gram-positive | S. aureus (NCTC 6538) | 32/64 | 2/4 | 2/2 | NA |
| MRSA (NCTC 12493) | 64/128 | 4/8 | 4/4 | 6.26 | |
| E. faecalis (NCTC 12697) | 64/128 | 8/8 | 8/8 | 12.53 | |
| Gram-negative | Escherichia coli (E. coli) (ATCC 8739) | 32/32 | 16/32 | 16/16 | NA |
| Klebsiella pneumonia (K. pneumoniae) (ATCC 43816) | 64/128 | 64/64 | 32/32 | 6.26 | |
| Pseudomonas aeruginosa (P. aeruginosa) (ATCC 9027) | 128/256 | 64/128 | 32/64 | NA | |
| HC50 b (µM) | 739.92 | 29.92 | 74.5 | NA | |
| Microorganisms | MBICs/MBECs (µM) | MBIC/MBEC (µg/mL) | |||
|---|---|---|---|---|---|
| B1OS | B1OS-L | B1OS-D-L | Ciprofloxacin a | ||
| Gram-positive | S. aureus (NCTC 6538) | 64/ > 512 | 4/128 | 4/128 | 1/ > 64 |
| MRSA (NCTC 12493) | 128/ > 512 | 8/128 | 4/128 | >64/ > 64 | |
| E. faecalis (NCTC 12697) | 256/ > 512 | 16/ > 512 | 8/ > 512 | NA | |
| Gram-negative | E. coli (ATCC 8739) | 128/512 | 64/256 | 32/128 | NA |
| K. pneumoniae (ATCC 43816) | 256/ > 512 | 128/ > 512 | 64/ > 512 | NA | |
| P. aeruginosa (ATCC 9027) | >512 | 256/ > 512 | 256/ > 512 | NA | |
| Cell Line | IC50 (µM) | ||
|---|---|---|---|
| B1OS | B1OS-L | B1OS-D-L | |
| H838 | 36.84 | 3.976 | 2.553 |
| PC-3 | 95.94 | 5.473 | 2.629 |
| U251MG | 233.0 | 8.629 | 3.492 |
| MCF-7 | 112 | 8.883 | 3.17 |
| HCT116 | 33.69 | 11.03 | 3.721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, A.; Ma, Y.; Chen, X.; Zhou, M.; Xi, X.; Ma, C.; Ren, S.; Chen, T.; Shaw, C.; Wang, L. Modification Strategy of D-leucine Residue Addition on a Novel Peptide from Odorrana schmackeri, with Enhanced Bioactivity and In Vivo Efficacy. Toxins 2021, 13, 611. https://doi.org/10.3390/toxins13090611
Yao A, Ma Y, Chen X, Zhou M, Xi X, Ma C, Ren S, Chen T, Shaw C, Wang L. Modification Strategy of D-leucine Residue Addition on a Novel Peptide from Odorrana schmackeri, with Enhanced Bioactivity and In Vivo Efficacy. Toxins. 2021; 13(9):611. https://doi.org/10.3390/toxins13090611
Chicago/Turabian StyleYao, Aifang, Yingxue Ma, Xiaoling Chen, Mei Zhou, Xinping Xi, Chengbang Ma, Shen Ren, Tianbao Chen, Chris Shaw, and Lei Wang. 2021. "Modification Strategy of D-leucine Residue Addition on a Novel Peptide from Odorrana schmackeri, with Enhanced Bioactivity and In Vivo Efficacy" Toxins 13, no. 9: 611. https://doi.org/10.3390/toxins13090611






