Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi
Abstract
:1. Introduction
2. Results
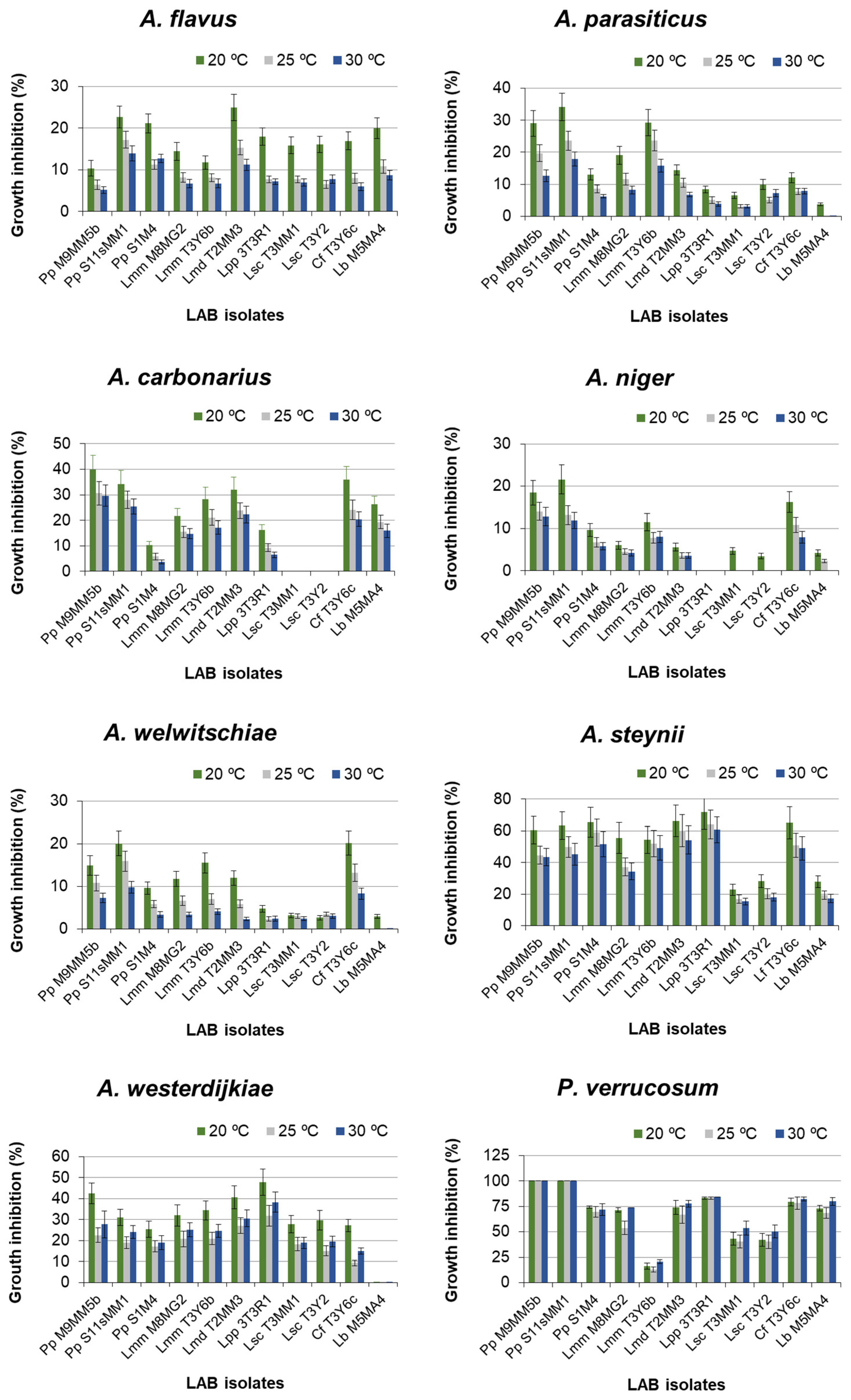
2.1. Effect of LAB on Fungal Growth in Dual Medium MRS-CYA20S
2.2. Machine Learning Approach to Model Fungal Growth Inhibition
2.3. Effect of the LAB Strains on Mycotoxin Production
2.3.1. Results of the Validation of the Method for Mycotoxin Determination
2.3.2. Mycotoxin Production in Controls and Cultures Containing LAB
- Aflatoxins
- 2.
- Ochratoxin A
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Standards
5.2. Microbial Strains and Culture Conditions
5.3. Antifungal Assays
5.3.1. Inoculum of Bacterial and Fungal Preparation
5.3.2. Preparation, Inoculation, and Incubation of Dual Cultures
5.4. Mycotoxin Determination
5.4.1. Calibration Solutions
5.4.2. Mycotoxin Recovery
5.4.3. Determination of Mycotoxins in Dual Cultures MRS-CYA20S
5.4.4. UPLC-MS/MS Conditions
5.5. Statistics
5.6. Method for the Design of Predictive ML Models for Growth Inhibition Percentage
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation List of the LAB Species
| Full Name | Abbreviation |
| Pediococcus pentosaceus | Pp |
| Leuconostoc mesenteroides ssp. mesenteroides | Lmm |
| Leuconostoc mesenteroides ssp. dextranicum | Lmd |
| Lacticaseibacillus paracasei ssp. paracasei | Lpp |
| Latilactobacillus sakei ssp. carnosus | Lsc |
| Companilactobacillus farciminis | Cf |
| Levilactobacillus brevis | Lb |
References
- European Commission. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 5–24. [Google Scholar]
- Marasas, W.F.O.; Gelderblom, W.C.A.; Shephard, G.S.; Vismer, H.F. Mycotoxins: A global problem. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; CABI: Wallingford, UK, 2008; pp. 29–40. [Google Scholar]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.-B.; Novakova, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenar, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Naeem, I.; Ismail, A.; Rehman, A.U.; Ismail, Z.; Saima, S.; Naz, A.; Faraz, A.; de Oliveira, C.A.F.; Benkerroum, N.; Aslam, M.Z.; et al. Prevalence of aflatoxins in selected dry fruits, impact of storage conditions on contamination levels and associated health risks on Pakistani consumers. Int. J. Environ. Res. Public Health 2022, 19, 3404. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Romera, D.; Mateo, E. M: Study on mycotoxin contamination of maize kernels in Spain. Food Control 2020, 118, 107370. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Martínez, R.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in breakfast cereals from Spanish market. Food Control 2011, 22, 1949–1955. [Google Scholar] [CrossRef]
- Bashiry, M.; Javanmardi, F.; Sadeghi, E.; Shokri, S.; Hossieni, H.; Oliveira, C.A.F.; Khaneghah, A.M. The prevalence of aflatoxins in commercial baby food products: A global systematic review, meta-analysis, and risk assessment study. Trends Food Sci. Technol. 2021, 114, 100–115. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pereira, J.L.; Fungaro, M.H.; Taniwaki, M.H. Aflatoxigenic fungi and aflatoxin in cocoa. Int. J. Food Microbiol. 2011, 148, 141–144. [Google Scholar] [CrossRef]
- Lutfullah, G.; Hussain, A. Studies on contamination level of aflatoxins in some cereals and beans of Pakistan. Food Control 2012, 23, 32–36. [Google Scholar] [CrossRef]
- Mollayusefian, I.; Ranaei, V.; Pilevar, Z.; Cabral-Pinto, M.M.S.; Rostami, A.; Nematolahi, A.; Khedher, K.M.; Thai, V.M.; Fakhri, Y.; Khaneghah, A.M. The concentration of aflatoxin M1 in raw and pasteurized milk: A worldwide systematic review and meta-analysis. Trends Food Sci. Technol. 2021, 115, 22–30. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Evaluation of Carcinogenic Risks of Chemicals to Humans. Some Naturally-Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In Aflatoxins: IARC Monographs; IARC: Lyon, France, 1993; Volume 56, pp. 245–395. [Google Scholar]
- Center for Disease Control and Prevention (CDC). Outbreak of Aflatoxin Poisoning—Eastern and Central Provinces, Kenya. 3 September 2004. Available online: https://www.cdc.gov/nceh/hsb/chemicals/aflatoxin.htm (accessed on 20 July 2022).
- Gil-Serna, J.; Patiño, B.; Cortes, L.; González-Jaén, M.T.; Vázquez, C. Aspergillus steynii and Aspergillus westerdijkiae as potential risk of OTA contamination in food products in warm climates. Food Microbiol. 2015, 46, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-B.; Lee, M.; Kim, D.-H.; Varga, J.; Frisvad, J.C.; Perrone, G.; Gomi, K.; Yamada, O.; Machida, M.; Houbraken, J.; et al. Aspergillus luchuensis, an industrially important black Aspergillus in East Asia. PLoS ONE 2013, 8, e63769. [Google Scholar] [CrossRef] [Green Version]
- Perrone, G.; Stea, G.; Epifani, F.; Varga, J.; Frisvad, J.C.; Samson, R.A. Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol. 2011, 115, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Amézqueta, S.; Schorr-Galindo, S.; Murillo-Arbizu, M.; González-Peñas, E.; López de Cerain, A.; Guiraud, J.P. OTA-producing fungi in foodstuffs: A review. Food Control 2012, 26, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Geisen, R.; Schmidt-Heydt, M.; Touhami, N.; Himmelsbach, A. New aspects of ochratoxin A and citrinin biosynthesis in Penicillium. Curr. Opin. Food Sci. 2018, 23, 23–31. [Google Scholar] [CrossRef]
- Kara, G.N.; Ozbey, F.; Kabak, B. Co-occurrence of aflatoxins and ochratoxin A in cereal flours commercialised in Turkey. Food Control 2015, 54, 275–281. [Google Scholar] [CrossRef]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Mateo, E.M.; Gil-Serna, J.; Patiño, B.; Jiménez, M. Aflatoxins and ochratoxin A in stored barley grain in Spain and impact of PCR-based strategies to assess the occurrence of aflatoxigenic and ochratoxigenic Aspergillus spp. Int. J. Food Microbiol. 2011, 149, 118–126. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Mateo, E.M. Potential Health Risk Associated with Mycotoxins in Oat Grains Consumed in Spain. Toxins 2021, 13, 421. [Google Scholar] [CrossRef]
- Mateo, R.; Medina, A.; Mateo, F.; Mateo, E.M.; Jiménez, M. An overview of ochratoxin A in beer and wine. Int. J. Food Microbiol. 2007, 119, 79–83. [Google Scholar] [CrossRef]
- Mehri, F.; Esfahani, M.; Heshmati, A.; Jenabi, E.; Khazaei, S. The prevalence of ochratoxin A in dried grapes and grape-derived products: A systematic review and meta-analysis. Toxin Rev. 2022, 41, 347–356. [Google Scholar] [CrossRef]
- Anelli, P.; Haidukowski, M.; Epifani, F.; Cimmarusti, M.T.; Moretti, A.; Logrieco, A.; Susca, A. Fungal mycobiota and mycotoxin risk for traditional artisan Italian cave cheese. Food Microbiol. 2019, 78, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, A.; Gasperini, A.M.; Copetti, M.V. Ecophysiology of OTA-producing fungi and its relevance in cured meat products. Review. Curr. Opin. Food Sci. 2022, 45, 100838. [Google Scholar] [CrossRef]
- Duarte, S.C.; Pena, A.; Lino, C.M. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef]
- Benites, A.J.; Fernandes, M.; Boleto, A.R.; Azevedo, S.; Silva, S.; Leitão, A.L. Occurrence of ochratoxin A in roasted coffee samples commercialized in Portugal. Food Control 2017, 73, 1223–1228. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Nester, M.A.; Efraim, P.; Taniwaki, M.H. Occurrence of ochratoxin A in cocoa by-products and determination of its reduction during chocolate manufacture. Food Chem. 2013, 136, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Bircan, C. Incidence of ochratoxin A in dried fruits and co-occurrence with aflatoxins in dried figs. Food Chem. Toxicol. 2009, 47, 1996–2001. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control 2014, 39, 192–197. [Google Scholar] [CrossRef]
- Khoi, C.S.; Chen, J.H.; Lin, T.Y.; Chiang, C.K.; Hung, K.Y. Ochratoxin A-Induced Nephrotoxicity: Up-to-Date Evidence. Int. J. Mol. Sci. 2021, 22, 11237. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E.H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [Green Version]
- Gan, F.; Zhou, Y.; Hou, L.; Qian, G.; Chen, X.; Huang, K. Ochratoxin A induces nephrotoxicity and immunotoxicity through different MAPK signaling pathways in PK15 cells and porcine primary splenocytes. Chemosphere 2017, 182, 630–637. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.W.; Venugopalan, L.P.; Beaussart, A.; Karnam, A.; Mohammed, M.R.S.; Jayapal, J.M.; Bretagne, S.; Bayry, J.; Prajna, L.; Kuppamuthu, D.; et al. Species-specific immunological reactivities depend on the cell-wall organization of the two Aspergillus, Aspergillus fumigatus and A. flavus. Front. Cell. Infect. Microbiol. 2021, 11, 643312. [Google Scholar] [CrossRef]
- Denning, D.M.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture (USDA). Grain, Fungal Diseases and Mycotoxin Reference; United States Grain Inspection, Packers and Stockyards Administration: Washington, DC, USA, 2016. Available online: https://www.ams.usda.gov/sites/default/files/media/FungalDiseaseandMycotoxinReference2017.pdf (accessed on 4 February 2022).
- Winter, G.; Pereg, L. A review on the relation between soil and mycotoxins: Effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 2019, 70, 882–897. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Spadaro, D.; Garibaldi, A. Containment of mycotoxins in the food chain by using decontamination and detoxification techniques. In Practical Tools for Plant and Food Biosecurity; Gullino, M., Stack, J., Fletcher, J., Mumford, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 8, pp. 163–177. [Google Scholar] [CrossRef]
- James, A.; Zikankuba, V.L. Mycotoxins contamination in maize alarms food safety in sub-Sahara Africa. Food Control 2018, 90, 372–381. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Dussort, P. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [Green Version]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Robinson, T.P.; Hugas, M.; Cocconcelli, P.S.; Richard-Forget, F.; Klein, G.; von Wright, A. Qualified presumption of safety (QPS): A generic risk assessment approach for biological agents notified to the European food safety authority (EFSA). Trends Food Sci. Technol. 2010, 21, 425–435. [Google Scholar] [CrossRef]
- Bangar, S.P.; Sharma, N.; Kumar, M.; Ozogul, F.; Purewal, S.S.; Trif, M. Recent developments in applications of lactic acid bacteria against mycotoxin production and fungal contamination. Food Biosci. 2021, 44, 101444. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [Green Version]
- Muhialdin, B.J.; Saari, N.; Hussin, A.S.M. Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules 2020, 25, 2655. [Google Scholar] [CrossRef]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Barratt, J.L.N.; Kennedy, P.J.; Kaufer, A.; Calarco, L.; Ellis, J.T. Machine learning and applications in microbiology. FEMS Microbiol. Rev. 2021, 45, fuab015. [Google Scholar] [CrossRef]
- Asurmendi, P.; Barberis, C.; Pascual, L.; Dalcero, A.; Barberis, L. Influence of Listeria monocytogenes and environmental abiotic factors on growth parameters and aflatoxin B1 production by Aspergillus flavus. J. Stored Prod. Res. 2015, 60, 60–66. [Google Scholar] [CrossRef]
- Adedokun, E.O.; Rather, I.A.; Bajpai, V.K.; Park, Y.H. Biocontrol efficacy of Lactobacillus fermentum YML014 against food spoilage moulds using the tomato puree model. Front. Life Sci. 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Bueno, D.J.; Silva, J.O.; Oliver, G.; Gonzalez, S.N. Lactobacillus casei CRL 431 and Lactobacillus rhamnosus CRL 1224 as biological controls for Aspergillus flavus strains. J. Food Prot. 2006, 69, 2544–2548. [Google Scholar] [CrossRef]
- Pereira, G.V.D.; Beux, M.; Pagnoncelli, M.G.B.; Soccol, V.T.; Rodrigues, C.; Soccol, C.R. Isolation, selection and evaluation of antagonistic yeasts and lactic acid bacteria against ochratoxigenic fungus Aspergillus westerdijkiae on coffee beans. Lett. Appl. Microbiol. 2016, 62, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, F.; Favilla, M.; De Bellis, P.; Sisto, A.; de Candia, S.; Lavermicocca, P. Antifungal activity of strains of lactic acid bacteria isolated from a semolina ecosystem against Penicillium roqueforti, Aspergillus niger and Endomyces fibuliger contaminating bakery products. Syst. Appl. Microbiol. 2009, 32, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Bustos, A.Y.; de Valdez, G.F.; Gerez, C.L. Optimization of phenyllactic acid production by Pediococcus acidilactici CRL 1753. Application of the formulated bio-preserver culture in bread. Biol. Control 2018, 123, 137–143. [Google Scholar] [CrossRef]
- Tarazona, A.; Mateo, E.M.; Gómez, J.V.; Gavara, R.; Jiménez, M.; Mateo, F. Machine learning approach for predicting Fusarium culmorum and F. proliferatum growth and mycotoxin production in treatments with ethylene-vinyl alcohol copolymer films containing pure components of essential oils. Int. J. Food Microbiol. 2021, 338, 109012. [Google Scholar] [CrossRef]
- Mateo, E.M.; Gómez, J.V.; Tarazona, A.; García-Esparza, M.Á.; Mateo, F. Comparative Analysis of Machine Learning Methods to Predict Growth of F. sporotrichioides and Production of T-2 and HT-2 Toxins in Treatments with Ethylene-Vinyl Alcohol Films Containing Pure Components of Essential Oils. Toxins 2021, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, A.; Mateo, E.M.; Gómez, J.V.; Romera, D.; Mateo, F. Potential use of machine learning methods in assessment of Fusarium culmorum and Fusarium proliferatum growth and mycotoxin production in treatments with antifungal agents. Fungal Biol. 2021, 125, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, J. Application of Artificial Neural Networks to Assess the Mycological State of Bulk Stored Rapeseeds. Agriculture 2020, 10, 567. [Google Scholar] [CrossRef]
- Panagou, E.; Kodogiannis, V. Application of neural networks as a non-linear modelling technique in food mycology. Expert Syst. Appl. 2009, 36, 121–131. [Google Scholar] [CrossRef]
- Hernández-Mendoza, A.; García, H.S.; Steele, J.L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009, 47, 1064–1068. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Nurul-Adilah, Z.; Than, L.T.L.; Mohd-Redzwan, S. The binding efficiency and interaction of Lactobacillus casei Shirota toward aflatoxin B1. Front. Microbiol. 2018, 9, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muaz, K.; Riaz, M.; Rosim, R.E.; Akhtar, S.; Corassin, C.H.; Gonçalves, B.L.; Fernandes-Oliveira, C.A. In vitro ability of nonviable cells of lactic acid bacteria strains in combination with sorbitan monostearate to bind to aflatoxin M1 in skimmed milk. LWT Food Sci. Technol. 2021, 147, 111666. [Google Scholar] [CrossRef]
- Kumara, S.S.; Bashisht, A.; Venkateswaran, G.; Hariprasad, P.; Gayathri, D. Characterization of novel Lactobacillus fermentum from curd samples of indigenous cows from Malnad Region, Karnataka, for their aflatoxin B1 binding and probiotic properties. Probiot. Antimicrob. Proteins 2019, 11, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.X.; Amaro, F.X.; Romero, J.J.; Pereira, O.G.; Jeong, K.C.; Adesogan, A.T. The capacity of silage inoculant bacteria to bind aflatoxin B1 in vitro and in artificially contaminated corn silage. J. Dairy Sci. 2017, 100, 7198–7210. [Google Scholar] [CrossRef]
- Fuchs, S.; Sontag, G.; Stidl, R.; Ehrlich, V.; Kundi, M.; Knasmüller, S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008, 46, 1398–1407. [Google Scholar] [CrossRef]
- Mateo, E.M.; Medina, A.; Mateo, F.; Valle-Algarra, F.M.; Pardo, I.; Jiménez, M. Ochratoxin A removal in synthetic media by living and heat-inactivated cells of Oenococcus oeni isolated from wines. Food Control 2010, 21, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Mateo, E.M.; Medina, A.; Mateo, R.; Jiménez, M. Effect of ethanol on the ability of Oenococcus oeni to remove ochratoxin A in synthetic wine-like media. Food Control 2010, 21, 935–941. [Google Scholar] [CrossRef]
- Piotrowska, M. The adsorption of ochratoxin A by Lactobacillus Species. Toxins 2014, 6, 2826–2839. [Google Scholar] [CrossRef] [Green Version]
- Belkacem-Hanfi, N.; Fhoula, I.; Semmar, N.; Guesmi, A.; Perraud-Gaime, I.; Ouzari, H.L.; Roussos, S. Lactic acid bacteria against post-harvest moulds and ochratoxin A isolated from stored wheat. Biol. Control 2014, 76, 52–59. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Taheur, F.B.; Mansour, C.; Kouidhi, B.; Chaieb, K. Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon 2019, 166, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Oliveira de Almeida-Moller, C.; Freire, L.; Rosim, R.E.; Pereira-Margalho, L.; Fasura-Balthazar, C.; Tuanny-Franco, L.; de Souza-Sant’Ana, A.; Corassin, C.H.; Rattray, F.P.; Fernandes de Oliveira, C.A. Effect of lactic acid bacteria strains on the growth and aflatoxin production potential of Aspergillus parasiticus, and their ability to bind aflatoxin B1, ochratoxin A, and zearalenone in vitro. Front. Microbiol. 2021, 12, 899. [Google Scholar]
- Ghanbari, R.; Aghaee, E.M.; Rezaie, S.; Khaniki, G.J.; Alimohammadi, M.; Soleimani, M.; Noorbakhsh, F. The inhibitory effect of lactic acid bacteria on aflatoxin production and expression of aflR gene in Aspergillus parasiticus. J. Food Saf. 2018, 38, e12413. [Google Scholar] [CrossRef]
- Gomaa, E.Z.; Abdelall, M.F.; El-Mahdy, O.M. Detoxification of aflatoxin B1 by antifungal compounds from Lactobacillus brevis and Lactobacillus paracasei, isolated from Dairy Products. Probiot. Antimicrob. Proteins 2018, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Taroub, B.; Salma, L.; Manel, Z.; Ouzari, H.I.; Hamdi, Z.; Moktar, H. Isolation of lactic acid bacteria from grapefruit: Antifungal activities, probiotic properties, and in vitro detoxification of ochratoxin A. Ann. Microbiol. 2019, 69, 17–27. [Google Scholar] [CrossRef]
- Elizaquível, P.; Pérez-Cataluña, A.; Yépez, A.; Aristimuño, C.; Jiménez, E.; Cocconcelli, P.S.; Vignolo, G.; Aznar, R. Pyrosequencing vs. culture-dependent approaches to analyze lactic acid bacteria associated to chicha, a traditional maize-based fermented beverage from Northwestern Argentina. Int. J. Food Microbiol. 2015, 198, 9–18. [Google Scholar] [CrossRef]
- Jiménez, E.; Yépez, A.; Pérez-Cataluña, A.; Ramos Vásquez, E.; Zúñiga Dávila, D.; Vignolo, G.; Aznar, R. Exploring diversity and biotechnological potential of lactic acid bacteria from tocosh—traditional Peruvian fermented potatoes—by high throughput sequencing (HTS) and culturing. LWT-Food Sci. Technol. 2018, 87, 567–574. [Google Scholar] [CrossRef]
- Magnusson, L.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 2003, 219, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Romera, D.; Mateo, E.M.; Mateo-Castro, R.; Gómez, J.V.; Gimeno-Adelantado, J.V.; Jiménez, M. Determination of multiple mycotoxins in feedstuffs by combined use of UPLC–MS/MS and UPLC–QTOF–MS. Food Chem. 2018, 267, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Benesty, M.; et al. Classification and Regression Training. Version 6.0-93. 2022. Available online: https://cran.r-projet.org.//web/pakages/caret/caret.pdf (accessed on 1 September 2022).


| Fungal Species | Homogeneous Groups 1 | |||||
|---|---|---|---|---|---|---|
| Lower ◄—Susceptibility—► Higher | ||||||
| A | B | C | D | E | F | |
| A. flavus | X | |||||
| A. parasiticus | X | |||||
| A. carbonarius | X | |||||
| A. niger | X | |||||
| A. welwitschiae | X | |||||
| A. steynii | X | |||||
| A. westerdijkiae | X | |||||
| P. verrucosum | X | |||||
| LAB Species (Strain) | Homogeneous Groups 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Lower ◄—Efficacy—► Higher | ||||||||
| A | B | C | D | E | F | G | H | |
| Pp (M9MM5b) | X | |||||||
| Pp (S11sMM1) | X | |||||||
| Pp (S1M4) | X | |||||||
| Lmm (M8MG2) | X | |||||||
| Lmm (T3Y6b) | X | |||||||
| Lmd (T2MM3) | X | |||||||
| Lpp (3T3R1) | X | |||||||
| Lsc (T3MM1) | X | |||||||
| Lsc (T3Y2) | X | |||||||
| Cf (T3Y6c) | X | X | ||||||
| Lb (M5MA4) | X | |||||||
| LAB Strain | Fungal Species | |||||||
|---|---|---|---|---|---|---|---|---|
| A. flavus | A. parasiticus | A. carbonarius | A. niger | A. welwitschiae | A. steynii | A. westerdijkiae | P. verrucosum | |
| Pp (M9MM5b) | 1 | 7 | 8 | 7 | 5 | 3 | 6 | 6 |
| Pp (S11sMM1) | 6 | 9 | 7 | 7 | 7 | 3, 4 | 4, 5 | 6 |
| Pp (S1M4) | 5 | 4, 5 | 2 | 4 | 3 | 4, 5 | 2, 3 | 4 |
| Lmm (M8MG2) | 2, 3 | 6 | 4 | 3 | 3 | 2 | 5 | 3 |
| Lmm (T3Y6b) | 2 | 8 | 5 | 5 | 4 | 3, 4 | 5 | 1 |
| Lmd (T2MM3) | 6 | 5 | 6 | 3 | 3 | 5, 6 | 6 | 4 |
| Lpp (3T3R1) | 3 | 2, 3 | 3 | 1 | 2 | 6 | 7 | 5 |
| Lsc (T3MM1) | 2, 3 | 2 | 1 | 2 | 2 | 1 | 3, 4 | 2 |
| Lsc (T3Y2) | 2, 3 | 3, 4 | 1 | 1, 2 | 2 | 1 | 3, 4 | 2 |
| Cf (T3Y6c) | 2, 3 | 4, 5 | 6, 7 | 6 | 6 | 3, 4, 5 | 2 | 5 |
| Lb (M5MA4) | 4 | 1 | 5 | 2 | 1 | 1 | 1 | 4 |
| ML Algorithm 1 | Assayed Parameters | Best Model Parameters | RMSE 2 | R-Squared |
|---|---|---|---|---|
| MLR | Regression coefficients | Found by the least-square method | 0.2714 | 0.7629 |
| MLP | size: 1–20; decay: 0.01, 0.05, 1.00 | size = 7; decay = 0.01 | 0.1999 | 0.9232 |
| RF | mtry: 2, 3; ntry: 500 | mtry = 3 | 0.2268 | 0.8623 |
| XGBoost | max-depth: 2–7; eta: 0.1–0.5); subsample: 0.5, 0.75, 1 | max_depth = 5, eta = 0.2, subsample = 1 | 0.2828 | 0.7767 |
| Mycotoxin 1 | Retention Time (min) | LOD (ng/g) | LOQ (ng/g) | Mean Recovery (%) | Mean RSD of Recoveries (%) |
|---|---|---|---|---|---|
| AFB1 | 8.30 | 0.78 | 2.34 | 85.2 | 9.5 |
| AFB2 | 8.04 | 0.8 | 2.4 | 87.3 | 11 |
| AFG1 | 7.80 | 1.18 | 3.5 | 83.0 | 8.2 |
| AFG2 | 7.50 | 0.4 | 1.2 | 90.5 | 8.7 |
| OTA | 10.52 | 0.8 | 2.4 | 91.1 | 7.6 |
| Fungi | |||||
|---|---|---|---|---|---|
| A. flavus | A. parasiticus | ||||
| Mycotoxin | Temperature (°C) | Minimum Reduction (%) | Maximum Reduction (%) | Minimum Reduction (%) | Maximum Reduction (%) |
| AFB1 | 20 | 32.7 | 52.3 | 20.0 | 55.0 |
| 25 | 22.8 | 37.9 | 19.7 | 44.7 | |
| 30 | 23.8 | 34.0 | 19.0 | 35.2 | |
| AFB2 | 20 | 32.9 | 57.0 | 20.7 | 55.3 |
| 25 | 16.2 | 38.3 | 21.5 | 45.5 | |
| 30 | 20.6 | 34.9 | 21.4 | 60.8 | |
| AFG1 | 20 | - | - | 21.2 | 59.7 |
| 25 | - | - | 22.4 | 44.4 | |
| 30 | - | - | 19.8 | 37.1 | |
| AFG2 | 20 | - | - | 25.0 | 59.7 |
| 25 | - | - | 22.1 | 45.3 | |
| 30 | - | - | 18.6 | 35.5 | |
| Fungal Species | Homogeneous Groups | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| A. carbonarius | X | |||||
| A. niger | X | |||||
| A. welwitschiae | X | |||||
| A. steynii | X | |||||
| A. westerdijkiae | X | |||||
| P. verrucosum | X | |||||
| Fungi | Temperature (°C) | OTA | |
|---|---|---|---|
| Minimum Reduction (%) | Maximum Reduction (%) | ||
| A. carbonarius | 20 | 22.6 | 55.6 |
| 25 | 23.7 | 52.1 | |
| 30 | 22.1 | 48.4 | |
| A. niger | 20 | 23.0 | 56.3 |
| 25 | 27.0 | 37.0 | |
| 30 | 7.3 | 51.2 | |
| A. welwitschiae | 20 | 28.8 | 77.9 |
| 25 | 27.9 | 39.3 | |
| 30 | 21.1 | 87.4 | |
| A. steynii | 20 | 44.5 | 94.8 |
| 25 | 39.2 | 81.8 | |
| 30 | 33.5 | 75.6 | |
| A. westerdijkiae | 20 | 18.7 | 61.1 |
| 25 | 25.6 | 53.2 | |
| 30 | 23.8 | 57.3 | |
| P. verrucosum | 20 | 36.7 | 100.0 |
| 25 | 27.0 | 100.0 | |
| 30 | 35.6 | 100.0 | |
| Mycotoxin | ESI Polarity | Molecular Mass (Da) | Precursor Ion | m/z (Da) | Product Ion (m/z) (Da) | DP(V) | EP(V) | CE(V) | CXP(V) |
|---|---|---|---|---|---|---|---|---|---|
| AFB1 | + | 312.063 | [M + H]+ | 313.1 | 285.2 1 | 106 | 10 | 33 | 16 |
| 128.1 2 | 106 | 10 | 91 | 10 | |||||
| AFB2 | + | 314.079 | [M + H]+ | 315.1 | 287.2 1 | 96 | 10 | 37 | 18 |
| 259.2 2 | 96 | 10 | 43 | 18 | |||||
| AFG1 | + | 328.058 | [M + H]+ | 329.1 | 243.1 1 | 86 | 10 | 39 | 14 |
| 200.0 2 | 86 | 10 | 59 | 12 | |||||
| AFG2 | + | 330.074 | [M + H]+ | 331.1 | 313.2 1 | 111 | 10 | 35 | 18 |
| 245.2 2 | 111 | 10 | 43 | 14 | |||||
| OTA | + | 403.082 | [M + H]+ | 404.0 | 239.0 1 | 91 | 10 | 37 | 16 |
| 102.0 2 | 91 | 10 | 105 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo, E.M.; Tarazona, A.; Jiménez, M.; Mateo, F. Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi. Toxins 2022, 14, 807. https://doi.org/10.3390/toxins14110807
Mateo EM, Tarazona A, Jiménez M, Mateo F. Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi. Toxins. 2022; 14(11):807. https://doi.org/10.3390/toxins14110807
Chicago/Turabian StyleMateo, Eva María, Andrea Tarazona, Misericordia Jiménez, and Fernando Mateo. 2022. "Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi" Toxins 14, no. 11: 807. https://doi.org/10.3390/toxins14110807
APA StyleMateo, E. M., Tarazona, A., Jiménez, M., & Mateo, F. (2022). Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi. Toxins, 14(11), 807. https://doi.org/10.3390/toxins14110807






