1. Introduction
The cornerstone of the mechanical theory of heat (MTH) is the equivalence principle (or, the mechanical equivalent of heat (MEH)): heat and mechanical work are equivalent. The crucial point here is about what the meaning of the word heat is. Clausius made the typical use of the word:
In all cases where work is produced by heat, a quantity of heat proportional to the work done is expended; and inversely, by the expenditure of a like quantity of work, the same amount of heat may be produced.
So, heat is expended heat; that is, heat causes the production of work; for this reason, steam engines became known as heat engines. This paper makes the case against this universally accepted understanding—and, with that, makes the case that MTH is a defective theory.
The extraordinary success of MTH was found in the introduction of energy by defining heat as a form of energy providing link between the science of motion (mechanics) and the science of heat. That link was of course one of the most consequential steps in science. This paper argues that defining heat as a form of energy, significant though it was as a move in support of reductionist philosophical mechanism, is in fact problematic: heat can only be comprehended in terms of both energy and entropy, or in terms of both the first law and the second law.
This is a conclusion drawn here from a review of the evolution of the second law thought from its beginning by Carnot to its incorporation by Thomson and Clausius into MTH, which was based on the ontology doctrine of heat as a form of energy. A critical assessment of MTH’s ontological commitment points to the conflict between that commitment and second law’s full development or the full expression. Even though Thomson and Clausius both viewed their MTH projects to be the synthesis of both Joule’s thesis that work “required the
consumption of heat” and Carnot’s thesis that work “required the
transfer of heat” [
2], by starting off with the definition of heat, that predisposition (commitment) precluded for their synthesis to do full justice to Carnot’s thesis. As a consequence we have an understanding of heat that is full of contradictions—like an adolescent in a contradictory and puzzling wonderland that only entropic insight provided by Carnot and Planck can guide its growth into adulthood.
To put the matter in another way, the theory of heat demands a choice: we must elect either to go with ontological commitment of defining what heat is—including full endorsement of the language police [
3]—or to go with the objective of gaining the full expression of the second law so that we have a coherent comprehension of heat, what can it do, what is its function, and how can we use it. That is, the choice of ontology purity, which necessarily hinders the full expression of the second law, or that of the full expression of the second law, which entails loosen-up of ontology purity. This paper lays out the latter choice—thermodynamics as a predicative, entropic theory of heat.
2. Carnot’s Caloric Flow
At its origins, thermodynamics was the study of heat and steam engines. Steam engines had been improved steadily from the time of Savery onwards by the eighteenth-century English engineers Newcomen, Smeaton, Watt, Woolf, and Trevithick. Those improvements were mostly empirical tinkering steps with the notable exception of Watt’s introduction of a separate condenser from the main engine cylinder so that alternate heating and cooling of the main cylinder was avoided—presciently reducing irreversibility due to heat transfer, a central concept to be formulated in thermodynamics. It was Sadi Carnot in 1824 [
4], however, who transformed the principle and operation of steam engines into a scientific discipline by posing “what, if any, are the limits to the efficiency of a steam engine?”
Whereas the laws of operation of machines with components of lever, pulley, gear, etc. that are powered manually or by animals, wind, or water were all well understood in terms of the mechanical theory based on the concept of force, there was no fundamental theory on the operation of machines powered by heat (i.e., heat engines). Carnot characterized the
force-driven (powered) mechanical theory of “machines which do not receive their motion from heat” a “complete theory”. Carnot’s genius manifested in his realization that “a similar theory is evidently needed for heat-engines…in the most general way the principle of the production of motion by heat…independently of any mechanism or any particular agent…whatever the working substance and whatever the method by which it is operated” [
4]. By explaining engine power in terms of
transfer of “caloric” from a hot body to a cold body Carnot provided a
“caloric”-driven general theory of heat engines. We shall call this theory Carnot’s theory of machines using heat (short as Carnot’s theory of heat or Carnot’s theory) as against the mechanical theory of machines not using heat; furthermore, we note that the former machines are driven, according to Carnot, by “caloric”, while the latter by force.
With regards to the meaning of “caloric”, there were two alternative interpretations: The first was, of course, the interpretation that caloric was a subtle fluid according to the caloric theory of heat; it was generally considered that that was how Carnot viewed caloric originally, all of which that left a hot body arrived at the lower temperature cold body without loss. The second, as it will be suggested below, was that “caloric flow” might be interpreted as entropy flow, the reason for its “conservation” in Carnot’s theory was that Carnot’s thought experiment dealt with reversible machines; a case will be made here that this interpretation serves a useful reading of Carnot’s theory in the development of the second law.
Carnot’s theory of heat may be summarized in terms of Carnot’s principle and Carnot’s function. Carnot’s principle is stated as:
No heat engine can be more efficient than a reversible one operating between the same temperatures.
A corollary of which is, then, “The motive power of heat is independent of the agents employed to realize it; its quantity is fixed solely by the temperatures of the bodies between which is effected, finally, the transfer of the caloric” [
4]. Expressing it mathematically, we have the inequality for work output
W of any real heat engine:
where the work output of the reversible heat engine is:
where
is caloric transferred, and
and
are the temperatures of the hot and cold bodies, respectively. With the principle, Carnot introduced a temperature function,
(called Carnot’s function), which is defined as a relation between the amount of heat given off by a source of heat transferred,
, between
t and
t − dt, and the reversible work,
, that can be derived from it:
He made several attempts to determine the temperature function and noted, “We do not know what laws it follows relative to the variations in volume: it is possible that its quantity changes either with the nature of the gas, its density, or its temperature. Experiment has taught us nothing on this subject” [
4] (pp. 16–17). Interestingly, it was the absence of the knowledge of the precise amount of caloric that gave him freedom to assume the values of
µ during step 6 and step 4 of the Carnot cycle. In one case he concluded that
µ is constant so that, since
is a conserved quantity in the caloric theory, integration of (3) yields:
where
Q* =
µQA. That is, “the motive power produced would be found exactly proportional to the fall of caloric” and the temperature difference. He, however, drew back from endorsing this special case afterward—realizing that he did not have the sound reason for this conclusion.
As we shall see below that derivation from (3) to (4) was successfully carried out later by Thomson. We can, therefore, view (4) and its interpretation by Carnot that the motive power produced would be exactly proportional to the fall of caloric as the essence of Carnot’s theory if “caloric” now is taken to be rather than the original . That is, the central premise of the theory is work to be derived from the transfer of “caloric” rather than its consumption: Carnot made it clear by noting, “Heat alone is not sufficient to give birth to the impelling power: it is necessary that there should also be cold; without it, the heat would be useless.” It may be noted for our discussion below that in that statement Carnot considered the triadic elements of “heat”, “cold”, and “power”—rather than the pair of heat and power (or, work).
It has been well established based on the fragments of his writings [
5] published posthumously by Hippolyte Carnot, Sadi’s brother, that Carnot had serious misgivings on heat as a subtle fluid and grappled with the question how, if he abandoned the substance theory of heat, could he reconcile the central tenet of his theory with its replacement: He was well aware of the competing idea of motive power to be derived from the
consumption of heat. Acceptance of that would contradict his presumption of heat conservation and, thus, he wondered aloud, “When motive power is produced by the passenger of heat from the body A to the body B, is the quantity of this heat which arrives at B the same whatever may be the substance employed to realize the motive power?” [
5]. But, he could not find satisfactory answer to the question, “Is there any way of using more heat in the production of motive power, and of causing less to reach the body B [at lower temperature]? Could we even utilize it entirely, allowing none to go to body B? If this were possible, motive power could be created… by mere destruction of the heat of bodies” [
5]. At the end, he concluded that accepting what will be called below the inter-convertibility principle would force him to abandon his theory of heat: “it would be difficult to explain why, in the development of motive power by heat, a cold body is necessary; why motion cannot be produced by consuming the heat of a warm body [alone]” [
5]. At the end, his commitment to the intrinsic necessity of the transfer or passage of heat for the production of work stood solid surviving his doubt of the substance theory of heat.
It will be suggested that Carnot’s intuition for what was right about his theory turns out to be correct. The ontological difficulty in the theory did not invalidate the theory because the presumption of heat as subtle fluid, or the presumption of heat conservation, was not crucial. His theory was not ontology based, was not about the nature of heat.
3. Thomson’s Resolution of Carnot’s Theory and Joule’s Doctrine, and His Energy Thinking
The idea in competition with Carnot’s theory that work is derived from the transfer of heat was the mechanical equivalent of heat (MEH) proposed by Mayer and Joule. Mathematically, it may be expressed as:
Joule was the principal investigator who provided the key empirical evidence for equivalence between expended work and produced heat [
6,
7]. He boldly, and correctly it turned out, proposed equivalence between disappeared heat and produced work as well.
Sometime in 1850 or 1851 Thomson began to realize that the acceptance of the equivalency (i.e., MEH) of heat and work did not have to discard what was essential in Carnot’s theory. He now adopted the view [
8] (pp. 174–220) and [
9] that heat dropped from a high to a low temperature in a reversible heat engine was, instead of being a conserved quantity, being continuously converted to work. If during a heat engine’s micro-cyclic-step an amount of heat
Q + δQ at
t + dt would descend to
Q at
t,
δQ of the heat would be converted to an equivalent amount of work
δW. That is:
The same work was calculated according to (3)
Though equation (6) had the same mathematical appearance as (3), there was a difference in their use: the original (3) was constrained with
QA to remaining constant but
Q in (6) is variable at different level of
t while heat is being converted into work in view of (5a). Note that (5a) is applicable to all processes including reversible processes. We now consider the combination of (5a) and (6) for a reversible process (but with subscript “
rev” removed from Equation (6)), which results in:
By rearranging the equation into:
Thomson arrived at, by integration, the result:
The complete heat engine cycle converted the heat
to the amount of work
. Thomson thus obtained in 1851 the result:
In 1854, Thomson ([
8], pp. 393–394) returned to his 1848 thermometry principle, which redefined the problem of Carnot’s function: Instead of the determination of
μ as a function of temperature, the realization that the function
determines (i.e.,
defines) an absolute temperature scale. Representing temperatures on this scale with
, thermometry principle asserts:
Substitution of which into (7) yields (because
):
This is of course the celebrated Carnot formula. It is justified for us to call it the
Carnot-Kelvin formula as Thomson (later, Kelvin) was the one who obtained the formula as well as established the concept of absolute temperature. In terms of usual notation of absolute temperature related to ideal gas thermometer, (9), with
W and
QA both in unit of joule, may be expressed as:
Thomson now saw similarity between dissipation of mechanical energy and heat transfer process. The latter case, if it took place without the production of work, amounted to a loss just like the mechanical energy dissipation. It was dissipation that seemed to Thomson to lie behind all the disparate unidirectional phenomena (…bulk energy had been dissipated into increased energy in microscopic motions). In an 1852 paper he ended with a terse declaration of three “general conclusions” ([
8], pp. 511–514):
GC1. “There is at present in the material world a universal tendency to the dissipation of mechanical energy.
GC2. “Any restoration of mechanical energy, without more than an equivalent of dissipation, is impossible…
GC3. “Within a finite period of time past, the earth must have been, and within a finite period of time to come the earth must again be, unfit for the habitation of man as at present constituted…”
These general conclusions taken together amounted to a declaration of universal dissipation of high grade energy. We have referred to them as
the energy principle [
10]. Note that the energy principle is
not the principle of conservation of energy. The principle of conservation of energy is directly linked to the idea of work production to be resulting from the
consumption of heat (MEH), whereas, in formulating the energy principle, Thomson attempted to bring Carnot’s idea of work production as resulting from the
transfer of heat to be consistent with MEH, the thesis of work production as resulting from the
consumption of heat. He was able to reconcile Carnot’s theory with Joule’s conflicting thesis in terms of energy solely: whereas Joule’s thesis was a doctrine of conservation of energy, Carnot’s was a doctrine of availability in energy that, though energy is never lost, its availability is being lost when heat transfers from high temperature to low temperature without attempt of work production. Thomson combined both doctrines into a doctrine of energy.
It is a remarkable fact that the energy doctrine of Thomson, or Kelvin’s energy doctrine, has been extraordinarily influential and, at the same time, the doctrine is based on questionable foundation—the foundation of universal dissipation of high grade energy, a principle that has been accepted as self-evident. We shall deal with the validity of this “self-evident truth” in the next section on the entropy principle.
4. The Entropy Principle
I have chosen in the section above to present Thomson’s contribution in the period of 1850-1854 in the resolution of MEH and Carnot’s theory instead of the simultaneous development by Clausius in the same period in the formulation of the first law and the second law, because Clausius went beyond his significant body of work in the period and made his greatest contribution when he introduced the concept of entropy in 1865.
Despite the great influence of Kelvin’s energy doctrine, everyone acknowledges the entropy principle, or the principle of the increase of entropy, to be the counterpoint to the great first law of thermodynamics. Clausius saw the necessity of going beyond the conception of energy after his attempts in a series of papers on “proof” of the so-called Clausius’ Inequality (of different versions), which culminated in his 1865 paper that introduced the concept of entropy: First of all, for a reversible cyclic process it was shown the existence of the equality:
It followed for a reversible change from state
A to state
B, a state function
S called
entropy can be defined:
For real cyclic process in general, an inequality known as Clausius’ inequality was shown to apply:
This inequality is often written as:
However, it was pointed out in a footnote in
Fermi’s Thermodynamics [
11], “
T’ represents the temperature of the source of the heat quantity
δQsystem that the Carnot engine surrenders to the system, and
is not, except in the reversibility limit,
equal to the temperature of the system (or any part of the system) along the quasi-static trajectory”. The applicability of the following inequality is questionable, therefore:
Nonetheless, for isolated systems or systems subject to adiabatic condition the inequality applies (because
vanishes, thus, the ambiguity of
T’ becomes irrelevant):
This is the celebrated principle of the increase of entropy, or the entropy principle:
For any transformation A→B occurring in an isolated system, the entropy of the final state can never be less than that of the initial state.
Unlike the energy principle, the universal validity of the entropy principle, universal entropy growth, can hardly be overstressed as its validity has been further supported—initiated by Maxwell, Boltzmann, and Gibbs—by the great body of work of statistical mechanics. Even though it has been argued that the entropy principle or the second law is only true statistically, not mathematically in the absolute sense—the statistical reasoning offers reassuring account for why a universe that is in the state of non-equilibrium-ness resulting from accelerating expansion should always move towards equilibrium (though never becomes equilibrium because of the moving equilibrium target) [
12,
13,
14,
15,
16].
With the introduction of entropy in terms of (11) and (11a), Carnot’s “caloric flow,”
, as it was suggested in the seventh paragraph of
Section 2, may be interpreted as entropy flow:
Because we are dealing with reversible heat flow, caloric, i.e., entropy flow, leaving body
A,
, arrives at body
B in the same amount,
. Therefore:
That is, the application of entropy-flow consideration to a reversible Carnot cycle is completely consistent with energy conservation consideration. What it was thought to be the requirement of heat conservation is really only the requirement of entropy “conservation,” i.e., absence of entropy growth in reversible Carnot cycle. In a significant sense, the introduction of entropy by Clausius validated Carnot’s intuition of falling entropy flow to be the cause of work production. In the following it will be argued that his intuition on the impossibility that “motive power could be created by mere destruction of the heat of bodies” was also supported by the fact that the second law could not be formulated in terms of energy alone.
That last point has been made by Planck, “the real meaning of the second law has frequently been looked for in a ‘dissipation of energy.’ This view, proceeding, as it does, from the irreversible phenomena of conduction and radiation of heat, presents only one side of the question…” [
17]. The intrigue question with the co-existence of the energy principle and the entropy principle was why it was necessary for Clausius to go beyond energy to formulate his second law. Dissipation of energy has served well (with appreciation to Kelvin) as a proxy to entropy growth (see
Figure 2 below). Correspondingly, energy systems (see definition in [
18]) have served as proxy to entropy growth potential (to be introduced below). Had dissipation of energy exhausted growth of entropy, there would have had no need of
entropy growth and
entropy growth potential as
independent concepts. The proxy would have been the real thing; but of course, it is not. Example that Kelvin’s GC-2 is false can be found in [
18] as well as a general discussion of the relationship between the energy principle and the entropy principle was presented in a 2014 paper [
19]. In conclusion, the “self-evident” principle of universal dissipation of high grade energy has been refuted: high grade energy dissipates spontaneously but not universally [
18,
19]. Only entropy grows universally; with this realization, the introduction of entropy should have been an equally consequential step in science as the introduction of energy.
But, I shall suggest that, in the mechanical theory of heat (MTH), the entropy principle stops short of being the true counterpoint to the great first law of thermodynamics. As a result, the journey of introducing entropy as an equal partnership with energy in the two great laws has been an incomplete project. In the following I shall identify the culprit.
5. The Mechanical Theory of Heat, and the Takeaways from the Carnot-Kelvin Formula
By 1850, Joule had succeeded to demonstrate equivalence between consumed mechanical work and produced heat [
6] and additionally proposed equivalence between disappeared heat and produced mechanical work. Equivalence principle (or, MEH) is not, however, inter-convertibility principle. Earlier in his private writing, Carnot had argued that motive power could not be created by mere destruction, or consumption, of the heat of bodies [
5].
What happened in 1850-1854 was that MEH was upgraded to the interconvertibility principle. Both Kelvin and Clausius (as influenced by Joule) considered the most important matter for their project to be the overthrown of the substance theory of heat: the establishment of the assertion that heat is a form of microscopic mechanical energy and the phenomenon of heat is completely specified or measured by its energy equivalent, as memorably captured by Clausius, “the kind of motion we call heat.” With that goal in mind Kelvin, in his authoritative and influential 1851 paper
On the dynamical theory of heat [
8] (pp. 174–220), opened with these fateful words, “…Considering it as thus established, that heat is not a substance, but a dynamical form of mechanical effect, we perceive that there must be an equivalence between mechanical work and heat, as between cause and effect.” That is, Kelvin argued that MTH was an ontology-based theory, that heat is a form of energy, and that it followed from the nature of heat the principle of inter-convertibility of heat and work: work causes heat as well as heat causes work.
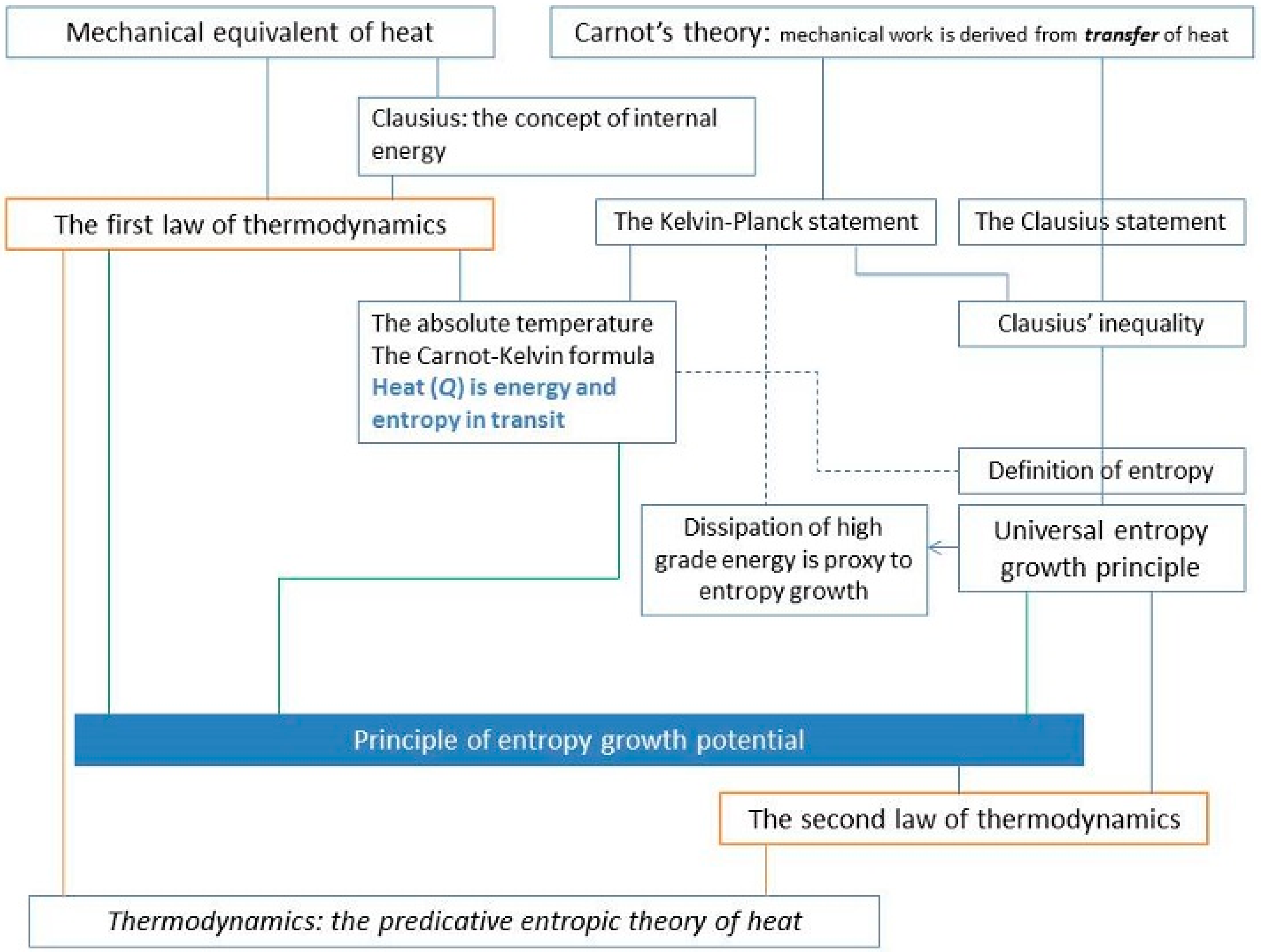
The key, in this reading of MTH as schematically shown in
Figure 1 (which is reproduced from [
18], is that work causes heat and, similarly, heat in and of itself causes work. That last point was what Carnot had difficulty with; he certainly did not deny the role of heat as falling caloric in the production of work but had difficulty with the idea of heat expenditure alone without transfer of heat as the cause for the production of work.
Many have questioned the validity of universal interconvertibility with regards to heat as a cause of work [
20,
21,
22,
23,
24,
25]. Yet, none of these arguments have succeeded in refuting universal inter-convertibility and the insistence of heat (i.e.,
Q) defined as energy in transit, rather than as energy and entropy in transit (as suggested in [
18]). Necessity in simultaneously refuting universal inter-convertibility and formulating a new theory of heat supplanting MTH has been made in a recent paper [
26], and will be recapitulated below. For further motivation why MTH should be critically reexamined, here in this section I shall discuss a number of specific difficulties in MTH. The principle example I use here is the Carnot-Kelvin formula as the most important tool in engineering thermodynamics.
Numerous thermodynamics instructors have advised students that, if they learn only one thing in thermodynamics, they should retain the Carnot-Kelvin formula and understand its takeaway. This is certainly a correct advice if the students get the right kind of take away. Unfortunately, it is almost impossible to have the right take away within the framework of MTH. Every student of thermodynamics bases her/his understanding of reversible processes on the perspective of (9a)
Correspondingly, he/she forms ideas roughly equivalent to the following general statements (GSs):
GS-1. It is impossible to extract work from a heat source without at the same time discarding a fraction of the heat.
GS-1.a. Heat, therefore, cannot be converted 100% into work.
GS-2. The lower the temperature of the heat sink the greater the work output is derived from heat of the heat source because of smaller fraction of the heat has to be discarded.
GS-2.a. Lower heat sink temperature is, therefore, intrinsically advantageous.
In the context of a Carnot cycle, of course, amount of heat must be discarded and, correspondingly, not all of can be converted into work, and the lower is the smaller the discarded heat will be, thus, the greater the work output is. GS-1 and GS-2 are true. Thinking in terms of the energy view-point of MTH, it seems that GS-1.a and GS-2.a are straightforward inference of GS-1 and GS-2 respectively.
Unfortunately, neither of which is an intrinsically true statement. In
Section 8, I shall explain why they are not true.
At this point, a (likely, an incomplete) list of erroneous statements in MTH resulting from its commitment to ontology purity is collected here:
GS-1.a. Heat cannot be converted 100% into work.
GS-2.a. Lower heat sink temperature is intrinsically advantageous.
GS-3. Heat by itself can cause mechanical work.
GS-4. As we never speak of “the work in a body,” we should never say “the heat in a body.”
GS-5. Change in infinitely-dense quasi-static processes is infinitely slow, thus, and apply to all quasi-static processes.
Of the five, GS-3 is the fundamental one as Joule and Kelvin identified (universal interconvertibility: “there must be an equivalence between mechanical work and heat, as between cause and effect”), one that the rest are results of which, directly or indirectly. As it will be shown in
Section 8, its replacement is necessary for transforming the entropy principle in MTH to becoming
the true counterpoint to the great first law of thermodynamics.
6. The Theory of Exergy
The theory of exergy was an important development in engineering thermodynamics by Kelvin, Gibbs, and Keenan, who correctly identified the production of work to be more than mere an energy issue but dependent on how an energy system approaching its equilibrium with its surroundings. As Dincer and Cengel noted:
The traditional method of assessing the energy disposition of an operation…is by the completion of an energy balance. This balance is apparently based on the first law of thermodynamics (FLT). In this balance, information on the system is employed to attempt to reduce heat losses or enhance heat recovery. However, from such a balance no information is available on the degradation of energy…The exergy method of analysis overcomes the limitations of the FLT. The concept of exergy is based on both FLT and second law of thermodynamics. Exergy analysis can clearly indicate the locations of energy degradation in a process that may lead to improved operation or technology…
The central feature the theory of exergy aims to capture is the feature of energy degradation. The notion of exergy or available energy went all the way back to Thomson (Kelvin) when he struggled to reconcile the conflict between Carnot and Joule in terms of understanding of energy, its conservation and its availability. In the literature, the introduction of the exergy (available energy) concept was often attributed to Josiah Willard Gibbs in 1873 [
28]. The concept was then, in the 1940s and 1950s, extended by Joseph Keenan for engineering applications, adapting it for the practical analysis of thermodynamic cycles, especially for power generation and refrigeration [
29]. The source of Gibbs’ insight, however, has been traced by Daub, as he noted in a historical study on “Entropy and Dissipation” to Thomson, “Although Gibbs never once mentioned Thomson in his work, he was indebted, I believe, to Thomson’s concept of dissipation of energy via the good offices of Maxwell and his
Theory of Heat. Maxwell, in turn, was indebted to Gibbs in the revision of his treatment of available and unavailable energy in his
Theory of Heat, thereby uniting the two traditions of entropy and dissipation” [
30] (p. 351). This reading of the history of thermodynamics is fully consistent with the historical study of Kelvin’s biographers, Smith and Wise [
31], who credit Kelvin to be the originator of the idea of available energy.
The concept of exergy deals with the “class of problems in engineering thermodynamics (concerning) systems or substances that can be modeled as being in (internal) equilibrium or stable (internal) equilibrium, but that are not in mutual stable equilibrium with the surroundings” [
27] (p. 129). It is necessary, thus, to define the surroundings or the environment, which according to Moran is assumed to be “large in extent and homogeneous in temperature (
) and pressure (
). All parts are at rest relative to one another. It is a source (or sink) of internal energy which can be freely drawn upon (or added to) without change in its intensive properties. It experiences only internally reversible processes in which the sole work mode is associated with volume change (
pdV work). It receives heat transfer of energy at the uniform temperature
” [
32] (p. 45).
Basic expression of exergy of an energy system may be obtained following Moran [
32] (pp. 46–48) and Moran and Shapiro [
33] (Chapter 7). Consider a combined system consisting of an energy system and the environment, only work interactions are permitted across the control surface of the combined system. Such work interaction,
(positive if it is produced work), can be stored in a work reservoir. Moreover, the total volume of the combined system remains constant.
The energy balance of the combined system is:
is the change in energy of the combined system
C, thus, the sum of the energy changes of the system and the environment. We have:
where
E is the energy of the system initially,
is the internal energy of the system in mutual equilibrium with the environment, and
is the change of energy in the environment as defined in the above. Under the defined conditions of the environment,
is:
Since the total volume of the combined system is constant, .
“Exergy is the maximum theoretical work obtainable from an overall system consisting of a system and the environment as the system comes into equilibrium with the environment” [
33] (p. 332). According to Carnot’s principle (or, the second law), maximum work is obtained if the change is reversible, i.e.,
. Substitution of
,
, and
into (15) yields:
Exergy is the maximum
, when
, i.e.,:
As Smith noted that Carnot’s engineering approach was followed by the North British tradition: “North British group of scientists and engineers, including James Joule, James Clerk Maxwell, William and James Thomson, Fleeming Jenkin, and P. G. Tait, developed energy physics to solve practical problems encountered by Scottish shipbuilders and marine engineers” [
34]. We may call this the Carnot-Joule-Kelvin tradition. With formulation of the entropy principle by Clausius, there has been a strong slant in thermodynamic theory towards pure science away from the Carnot-Joule-Kelvin tradition. It is important to acknowledge the great significance of the theory of exergy in keeping Kelvin’s contribution relevant and up-to-date today and keeping alive historical connection toward practical applications as well as injecting an intellectual rigor to engineering discourse in thermal engineering.
However, this brief review of exergy points out the limitations of exergy-based engineering thermodynamics: Unlike the concept of energy or entropy, their universal natures are captured by the first law and the second law, the concept of exergy does not enjoy the status of universality. (16) is applicable to simple systems in internal equilibrium, not to composite systems with tendency towards internal equilibrium; for complicated systems, alternative expressions have to be derived in their handling [
32].
In other words, as it was stated that the objective of introducing exergy is to take into consideration of energy degradation in addition to energy balance in any change, the demonstration that energy dissipation is spontaneous not universal should lead to the conclusion that exergy dissipation is not universal. If this prediction is proven to be true, the solidity in the foundation for the theory of exergy will be gone just as universal energy dissipation has been refuted.
Most importantly, the theory of exergy is a theory entirely within the framework of MTH. As such, its goal is not in providing full expression to the second law so that MTH can be repaired of all its self-contradictions: none of the erroneous statements in MTH, GS-1.a, GS-2.a, GS-3, GS-4 and GS-5, has been refuted in the development of the theory of exergy. As it will be shown that, by going outside the box of MTH, these erroneous statements will be corrected in
Section 8 and [
18].
7. The Entropy Growth Potential Principle
The telling passage in MTH is what Thomson stated as the starting point of his 1851 paper, “heat is not a substance, but a dynamical form of mechanical effect, we perceive that there must be an equivalence between mechanical work and heat, as between cause and effect” [
8] (pp. 174–220). By that, he made equivalence principle, which originally was a simple quantitative relation between heat and work, into a causal relation. Correspondingly, Thomson as well as Clausius considered their project to be the development of an ontology-based theory of heat based on the precept that heat is a form of energy. Even with the formulation by Clausius of the great principle of the increase of entropy, their commitment to the ontological purity prevented the entropy principle to become the true counterpoint to the principle of conservation of energy.
The culprit is the universal interconvertibility principle’s causal-relation interpretation. It is correct to view that work expended causes produced heat. But, the real reason that expended work leads to produced heat is an inference from universal entropy growth. To think in terms of heat expended to be the cause for the production of work as a matter of energetic causal relation without explicit use of the idea of universal entropy growth amounts to give heat the sole role of causing work. It is a role which heat cannot fulfill [
24]. Because of this incorrect assignment of role for heat, universal interconvertibility principle deprives the second law of its constructive function. MTH’s second law, as the law of universal entropy growth, is, consequently, incomplete.
Conceptual Differentiation of Entropy Growth and Entropy Growth Potential
The project of completing the second law is made possible on the basis of three recent works [
16,
18,
19]. There, the same kind of combined system consisting of a system and its environment used in exergy analysis was adopted; the combined system was referred to as “universe”; a work reservoir was explicitly introduced [
16]. The initial and final states of the system were given in the consideration. As Poincaré (1913) observed on the meaning the thermodynamic laws:
(These thermodynamic laws) can have only one significance, which is that there is a property common to all possibilities; but in the deterministic hypothesis there is only a single possibility, and the laws no longer have any meaning. In the indeterministic hypothesis, on the other hand, they would have meaning, even if they were taken in an absolute sense; they would appear as a limitation imposed upon freedom”.
There are, therefore, infinite possibilities in events for the system changing from the initial state to the final state as bounded by the spontaneous event and the reversible event. These two bookend-events define the range of possibilities, called the
Poincaré range [
18].
It is clear that the spontaneous event is characterized by positive entropy growth in the universe:
Equation (17), of course, is the same equation as (14) since the universe is by definition an isolated combined system. It was shown in [
16] that in the reversible event for a system interacting with its environment (heat reservoir at
), reversible work output is derived from heat extracted from the
heat reservoir of the amount:
That is, whereas in (17) entropy growth,
, stands originally for entropy generation as manifestation of dissipative spontaneous processes in the universe, here we obtain a result signifying a different role for the same term, the production of reversible work manifesting the constructive role of “entropic” events in the universe. Rather than
entropy Growth as it is originally called, the term in the context in the equation should be more properly called by the name of
entropy growth Potential (EGP), thus:
Change of the erstwhile subscript
G to
P in (19) signifies this fundamental change in the meaning of the 2nd law from the
law of dissipation to be the
primary premise that entropy growth potential is the driver in the huge manufactory of natural processes. As it has been noted in [
36] (p. xiv), “Irreversible processes are creators and destroyers of order,” a precept of the Brussels school of thermodynamics.
Equation (19) applies to systems in interaction with its heat reservoir. This consideration has been generalized to isolated systems with tendency towards internal equilibrium. The expression of reversible work for harnessing the EGPs of isolated systems when they are in interaction with given
-reservoir is [
19]:
Note that the entropy increase of spontaneous changes in an isolated system,
, is independent of any reservoir (thus, any reservoir temperature), whereas, in the case of (19),
is dependent on the value of
. In other words, while EGPs of isolated systems are independent of any reservoir, EGPs of systems interacting with their reservoirs are dependent on the temperature of their reservoirs (see
Section 8.3 below). In contrast to (19), reversible work to be derived from isolated∙system-reservoir, in (20), equals the product of
fixed and
arbitrary (i.e., variable)
.
Taking together (19) and (20), the erstwhile spontaneous entropy growth becomes EGP serving as the drive for reversible work as well as every event in the Poincare range. Every event in the Poincare range shares the same EGP, , but each specific event’s entropy growth, , is different:
For the aforementioned spontaneous event, .
For the reversible event, .
For a specific event, its entropy growth falls between the two limits:
Particular value of an event’s entropy growth depends on whether the event is a natural event or an event maintained by mechanism and, in the latter case, the operational principle of the mechanism. That is, EGP is the constructive drive potential giving rise to:
maximum reversible work if perfect control mechanism is provided;
valuable work if a real-world heat engine operational mechanism is provided; or
moderate amount of kinetic and potential mechanical energy in mechanism-free natural convective fluid bodies.
In sum, the full expression of the second law may be articulated by adding to universal entropy growth principle the principle of entropy growth potential:
No change in nature happens without entropy growth potential (see
Figure 2).
The principle of entropy growth potential serves as the cornerstone of a new theory of heat. A case can be made that Carnot’s theory (and consequently thermodynamics) is a predicative theory of heat. An ontological theory of heat attempts to answer, in the most general possible terms, the question, “What is heat?” A predicative theory of heat, instead, attempts to answer the question, “What is it to say something about heat?” An important question for a predicative theory is its category [
37].
The caloric theory is a one-place-relation theory that heat is conserved. Since Carnot addressed the problem of heat and work, Carnot’s theory, as a predicative theory of higher place framework, is not a caloric theory. Their focus on the ontological nature of heat prevented Kelvin and Clausius to understand that fact of Carnot’s theory. In fact by arguing, “Heat alone is not sufficient to give birth to the impelling power: it is necessary that there should also be cold; without it, the heat would be useless,” Carnot laid out a framework of the triad of heat, cold and power—an imperfect version of
EGP (in the hot and cold pair),
heat from reservoir and
power. [
16,
18,
19] The real innovation of Carnot’s theory is that Carnot formulated a model-based predicative theory instead of an ontology-based mechanical theory—and the triadic framework of his predicative theory, not its conservation of caloric (which turned out to be corresponding to the “conservation” of entropy for reversible processes), is the core essence of the theory.
8. The Triadic Framework and Heat Extraction
Sample applications of the principle of entropy growth potential can be found in [
18], including why GS-4 and GS-5 are false. This paper limits its discussion to demonstrate falsity of GS-1.a, GS-2.a, and GS-3 in the following.
8.1. GS-3_Universal Inter-Convertibility?
Thomson thought that universal inter-convertibility followed necessarily from the ontological doctrine that heat is a form of energy. As a referee of Joule’s 1850 paper pointed out that universal inter-convertibility did not follow from MEH (cited in [
7]), universal interconvertibility does not follow from the ontology of heat either. If one takes the second law seriously (which we do today more than Thomson and Clausius did because even they could not anticipate the profundity of the principles they created), the law must be understood by differentiating conceptually entropy growth and entropy growth potential. The latter concept entails a theory of heat, i.e., understanding energy transformation in the general sense, in the triadic framework.
Conversion of mechanical work into heat is spontaneous and, in such spontaneous process, entropy growth realizes and entropy growth potential is consumed—we can understand such a specific process in a dyadic framework. Examples of more detailed description of such a process can be found in [
18].
In the opposite direction, heat transfer from a hot body to a cold body also takes place spontaneously, in which entropy growth realizes and entropy growth potential is consumed. Similarly, in natural convection processes a small fraction of entropy growth is not realized in the form of moderate amount of kinetic and potential mechanical energy until their eventual dissipation. On the other hand, in the same direction conversion of heat into mechanical work can take place other than spontaneously. In an engineering conversion of heat into mechanical work, entropy growth and entropy growth potential must be differentiated: In a reversible conversion, only entropy growth potential is consumed with no entropy growth, while in a real engineering conversion entropy growth potential is consumed with partial entropy growth. In both, entropy growth is delayed or partially delayed, and its eventual realization occurs when all work is dissipated or when all orders created by work collapse at some point in the future. In all cases, the ultimate ending is always the same: all entropy growth potentials turn into dissipated heat eventually. The richness in this understanding is apprehended only in the triadic framework.
In the triadic framework, mechanical work is extracted from heat reservoir as based on MEH, but extraction is always powered by entropy growth potential. So is the conversion of work into heat or simple heat transfer processes. Regardless whether a process is spontaneous and can be understood in dyadic framework or one requires, for its happening, management in triadic framework, no process or change in nature happens without entropy growth potential. Certainly, no conversion of heat into mechanical work is possible without entropy growth potential: universal inter-convertibility of heat and mechanical work is demonstrably false.
The defunct universal inter-convertibility as the cornerstone of the theory of heat is replaced by the principle of entropy growth potential (compare
Figure 1 and
Figure 2).
8.2. GS-1.a
The very idea that heat is responsible for the production of mechanical work is the origin for the idea that heat cannot be converted 100% into mechanical work. Once we understand that mechanical work is not caused by heat (used in the narrow sense in universal inter-convertibility, not as
as used in [
18] and in
Figure 2), heat will no longer bear the responsibility nor be subject to the limitation.
In the dyadic framework of MTH, heat (consumption of heat) and entropy growth potential (degradation of energy in the form of transfer of heat) are conflated into a singular concept, the consumption and transformation of high grade energy (or, high grade heat). With such conflation of two independent entities, heat or energy cannot be consumed without at the same time be degraded. No matter how high is its grade, heat is subject to the condition of GS-1.a:
•
GS-1.a. Heat cannot be converted 100% into work.
However, in the triadic framework, spontaneity (synonymous with entropy growth potential) and heat, no longer automatically conflated into one, can be independent entities; dissipation of high grade energy is only one example of unidirectional processes while there are other examples of unidirectional processes involving pure entropy growth potential (i.e., pure spontaneity) with no degradation of energy; when such entropy growth potentials are available involving no degradation of energy (which is possible because it is not automatically conflated with heat or energy), heat from the heat reservoir alone is converted into work: rather than GS 1.a, which is a relic of MTH, we have this understanding, instead,
•
GS-1.b. Heat from a heat reservoir is converted 100% into work all the times as dictated by MEH; what is of special interest is that conversion of heat into mechanical work is possible even without degradation of energy in the consumption of entropy growth potential, i.e., with pure spontaneity.
One example of pure entropy growth potential is reversibly controlled
gaseous free expansion, which was cited by Fermi as example of why GS-1.a is not true [
11] (p. 30, in a footnote). Other examples have been and can be readily constructed as reported in many papers (including [
18]).
Note on the subsumption of the energy principle under the 2nd law: The energy principle is one interpretation of the second law in that entropy growth potential and heat are conflated into one resulting in leaving out the possibility of pure entropy growth potential involving no energy degradation. Such limited view can be captured in a dyadic framework with the full richness in the entropy principle lost. It is in this sense that Kelvin’s energy principle, GC1, GC2 and GC3, is subsumed under the second law in the triadic framework.
8.3. GS-2.a
Every student of thermodynamics knows that work increases with decreasing
of the heat sink in a Carnot engine. This is, however, not obvious in (19), which prima facie indicates
to be decreasing with decreasing
. The real reason for
’s ascending dependency on decreasing
is strong-increase in
, resulting from decrease in
:
This is why Carnot said that in addition to hot body “it is necessary that there should also be cold”. In this particular case, the cold reservoir serves as both the heat reservoir (so that heat can be extracted from) and heat sink (forming the hot-cold (source-sink) pair with entropy growth potential).
Greater increase, as shown in (22), in entropy growth potential of the hot-cold pair
overcompensates decrease in
itself, resulting in greater useful work even with smaller heat extracting capacity in a lower
, or
, heat reservoir:
It is not due to any intrinsic advantage in the lower temperature per se of a heat reservoir.
Equation (20) exposes the falsehood of GS-2.a as a general statement, while GS-2 is a simple statement of fact in a particular case. Better interpretation of GS-2 can be made once we move away from viewing the matter to be necessity in rejecting heat to the all-encompassing view of the matter to be the extraction of heat from reservoir driven by spontaneity as represented in (19) and (20). That is, GS-2 is, for avoiding the wrong implication of GS-2.a, better stated as:
•
GS-2.b. The lower the temperature of the heat sink the greater the work output is obtained because of resulting source-sink potential, which overcompensates the linearly weakening heat extracting capacity of the heat reservoir (see (19) and (20)) with faster strengthening heat extraction drive (as in (22)).
Work output depends on both entropy growth potential and the temperature of the reservoir; there is no intrinsic advantage of a lower reservoir temperature unless entropy growth potential is a strong function of the temperature of the reservoir doubling as the sink. This together with the above
subsection 8.2 gives us a definite understanding on the take-away of the Carnot cycle.
A schematic representation of the predicative entropic theory of heat is shown in
Figure 2 (reproduced from [
18]), in which the block of universal interconvertibility in
Figure 1 is replaced by the block of principle of entropy growth potential.
9. What is Expended vs. “What We Have to Pay to Get It”
The predicative entropic theory achieves the successful conclusion of Kelvin-Clausius’ synthesis-project: Equivalence exists between extracted heat and mechanical work, not expended heat and mechanical work. In the production of mechanical work neither heat nor energy is expended, heat is not because heat cannot cause work and energy is not because energy is never expended only the form of which can be changed, i.e., degraded. What is expended is EGPs.
In a series of papers that was summarized in a 2012 article [
38], Bizarro treated the effect of friction in the operation of idealized heat engine cycles. Friction of course reduces work output. The subtlety in Bizarro’s treatment was in taking into consideration of the effect of friction on “heat”
([
38]’s notation), which was defined as “what we have to pay to get it?” Bizarro’s treatment was an improvement within the framework of Kelvin’s energy principle, which conflates EGP with heat released from an energy system, i.e., conflates what is expended with what we pay for.
In the predicative entropic theory energy systems are identified with
stock EGP, which is one kind of EGPs. There is, thus, another kind of EGPs, namely
ongoing or
natural EGP [
18]. The expenditure of both kinds is important to the operation of modern industry and society. But, how we pay for their expenditures is different: We only pay for the former in terms of its quantity. What we pay for the expenditure of the latter is its management [
18].
10. Concluding Remark on the Completion of KELVIN-CLAUSIUS’ Synthesis-Project
Hardin made the case for the threat of “clarity” in language [
39]. “Traditional language always
seems clear. There seems to be great clarity in such sentences as these:
Heat flows. Life left him. He is possessed of a devil. He has a disease. He has a neurosis. But, for all their apparent clarity, they are surely all wrong. Their categories are wrong. All of them assert false substantives, when the discussion should be couched in terms of processes” [
39] (p. 395). But, the matter is not so unequivocal as Hardin himself admitted and a case can be made that when physicists and engineers mention
heat flows they are really talking about
flows (see [
18] for a discussion on the entropic meaning of
). In contrast, the phrase of “expenditure of heat” is a clear example of mistaken category: mistaking the three-place-relation of extraction of heat with the two-place-relation of expenditure of heat.
The introduction of entropy was an equally significant step for society and industry as the introduction of energy was. Its historical significance has not been fully realized because Thomson and Clausius as well as those who critiqued Thomson-Clausius’ MTH—Callendar, Job, Falk, Sestak [
40], Mares, and other far-sighted scientists and engineers—failed to appreciate that Carnot’s theory was not a caloric theory of heat. His was not an ontology-based theory, but a predicative theory. In this article I have presented an updated version of such a theory on the basis of entropy growth potential principle. With the principle as its cornerstone, Kelvin-Clausius’ synthesis-project is complete and thermodynamics becomes a coherent conceptual system.
Bill Gates commented on the importance of innovation in energy technologies, “As with cancer research, there’s a lot of uncertainty as to what scientific possibilities are out there. We don’t have a good equation” [
41]. Gates is correct that we need to explore unchartered territory of possibilities in order to solve the problem of energy and climate change, and he cites examples in chemistry and materials science. Recasting the problem rather than as an energy problem to be an entropy problem is another way to enlarge what is possible by fundamentally reframing the problem. As Emden eloquently wrote, “In the huge manufactory of natural processes, the principle of entropy occupies the position of manager, for it dictates the manner and method of the whole business, whilst the principle of energy (conservation) merely does the bookkeeping, balancing credits and debits”, [
42] thinking entropy makes the problem to be a supply-demand as well as a management problem [
43], rather than solely a supply-demand problem. What we pay for the supply of stock EGPs is different from what we pay for managing natural EGPs.