Human Postural Control: Assessment of Two Alternative Interpretations of Center of Pressure Sample Entropy through a Principal Component Factorization of Whole-Body Kinematics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Procedures
2.3. Data Analysis—COP Data Pre-Processing and Principal Component Analysis (PCA)
2.4. Calculation of Sample Entropy
2.5. Statistics
3. Results
4. Discussion
4.1. Main Results
4.2. Limitations
5. Conclusions and Outlook
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.; Stergiou, N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br. J. Sports Med. 2005, 39, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.S.; Stergiou, N. Recovery of postural control after cerebral concussion: New insights using approximate entropy. J. Athl. Train. 2006, 41, 305–313. [Google Scholar] [PubMed]
- Cavanaugh, J.T.; Mercer, V.S.; Stergiou, N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: A methodological report. J. Neuroeng. Rehabil. 2007, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, J.; Buckley, T.; White, K.; Hass, C. Shannon and Renyi entropies to classify effects of Mild Traumatic Brain Injury on postural sway. PLoS ONE 2011, 6, e24446. [Google Scholar] [CrossRef] [PubMed]
- Senthinathan, A.; Mainwaring, L.M.; Hutchison, M. Heart Rate Variability of Athletes across Concussion Recovery Milestones: A Preliminary Study. Clin. J. Sport Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Fournier, K.A.; Amano, S.; Radonovich, K.J.; Bleser, T.M.; Hass, C.J. Decreased dynamical complexity during quiet stance in children with autism spectrum disorders. Gait Posture 2014, 39, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.P.; Seigle, B.; Frère, J.; Parietti-Winkler, C. Dynamical analysis of balance in vestibular schwannoma patients. Gait Posture 2017, 54, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Busa, M.A.; Jones, S.L.; Hamill, J.; van Emmerik, R.E.A. Multiscale entropy identifies differences in complexity in postural control in women with multiple sclerosis. Gait Posture 2016, 45, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Busa, M.A.; Ducharme, S.W.; van Emmerik, R.E.A. Non-Linear Techniques Reveal Adaptive and Maladaptive Postural Control Dynamics in Persons with Multiple Sclerosis. J. Mult. Scler. 2016, 3, 1–3. [Google Scholar] [CrossRef]
- Lakhani, B.; Mansfield, A. Visual feedback of the centre of gravity to optimize standing balance. Gait Posture 2015, 41, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, M.; Ikezoe, T.; Kamiya, M.; Masaki, M.; Ichihashi, N. Correlation between movement complexity during static standing and balance function in institutionalized older adults. Clin. Interv. Aging 2017, 12, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, D.; Snyder, K.; Molfese, V.; Kyvelidou, A. Postural control strategies differ in normal weight and overweight infants. Gait Posture 2017, 55, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, S.; Seigle, B.; Lagarde, J.; Bouchara, F.; Bernard, P.L. On the use of sample entropy to analyze human postural sway data. Med. Eng. Phys. 2009, 31, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Leung, H.; Yue, L.; Deng, L. Evaluating Human Motion Complexity Based on Un-Correlation and Non-smoothness. In Advances in Multimedia Information Processing—PCM 2010; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2010; pp. 538–548. [Google Scholar]
- Chen, M.-S.; Jiang, B.C. Resistance Training Exercise Program for Intervention to Enhance Gait Function in Elderly Chronically Ill Patients: Multivariate Multiscale Entropy for Center of Pressure Signal Analysis. Available online: https://www.hindawi.com/journals/cmmm/2014/471356/ (accessed on 9 October 2017).
- Todorov, E.; Jordan, M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002, 5, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.; O’Hare, N. A quality control procedure for force platforms. Physiol. Meas. 2000, 21, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Goldie, P.A.; Bach, T.M.; Evans, O.M. Force platform measures for evaluating postural control: Reliability and validity. Arch. Phys. Med. Rehabil. 1989, 70, 510–517. [Google Scholar] [PubMed]
- Federolf, P.A. A novel approach to study human posture control: “Principal movements” obtained from a principal component analysis of kinematic marker data. J. Biomech. 2016, 49, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Daffertshofer, A.; Lamoth, C.J.C.; Meijer, O.G.; Beek, P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004, 19, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Troje, N.F. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. J. Vis. 2002, 2, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.; Tecante, K.; Nigg, B. A holistic approach to study the temporal variability in gait. J. Biomech. 2012, 45, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Gløersen, Ø.; Myklebust, H.; Hallén, J.; Federolf, P. Technique analysis in elite athletes using principal component analysis. J. Sports Sci. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bockemühl, T.; Troje, N.F.; Dürr, V. Inter-joint coupling and joint angle synergies of human catching movements. Hum. Mov. Sci. 2010, 29, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Verrel, J.; Lövdén, M.; Schellenbach, M.; Schaefer, S.; Lindenberger, U. Interacting effects of cognitive load and adult age on the regularity of whole-body motion during treadmill walking. Psychol. Aging 2009, 24, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.; Roos, L.; Nigg, B.M. Analysis of the multi-segmental postural movement strategies utilized in bipedal, tandem and one-leg stance as quantified by a principal component decomposition of marker coordinates. J. Biomech. 2013, 46, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.; Torres, A.; Sarlabous, L.; Jané, R. Influence of Parameter Selection in Fixed Sample Entropy of Surface Diaphragm Electromyography for Estimating Respiratory Activity. Entropy 2017, 19, 460. [Google Scholar] [CrossRef]
- Kanekar, N.; Lee, Y.-J.; Aruin, A.S. Frequency analysis approach to study balance control in individuals with multiple sclerosis. J. Neurosci. Methods 2014, 222, 91–96. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.; Turkman, A.; Swash, M. Motor unit firing in amyotrophic lateral sclerosis and other upper and lower motor neurone disorders. Clin. Neurophysiol. 2012, 123, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, J.; Enoka, R.M. Human motor unit recordings: Origins and insight into the integrated motor system. Brain Res. 2011, 1409, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Duclos, Y.; Burnet, H.; Schmied, A.; Rossi-Durand, C. Approximate entropy of motoneuron firing patterns during a motor preparation task. J. Neurosci. Methods 2008, 172, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Hodson-Tole, E.F.; Wakeling, J.M. Movement Complexity and Neuromechanical Factors Affect the Entropic Half-Life of Myoelectric Signals. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Busa, M.A.; van Emmerik, R.E.A. Multiscale entropy: A tool for understanding the complexity of postural control. J. Sport Health Sci. 2016, 5, 44–51. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Wei, Q.; Shieh, J.-S.; Fourcade, P.; Isableu, B.; Majed, L. Sample Entropy, Univariate, and Multivariate Multi-Scale Entropy in Comparison with Classical Postural Sway Parameters in Young Healthy Adults. Front. Hum. Neurosci. 2017, 11, 206. [Google Scholar] [CrossRef] [PubMed]
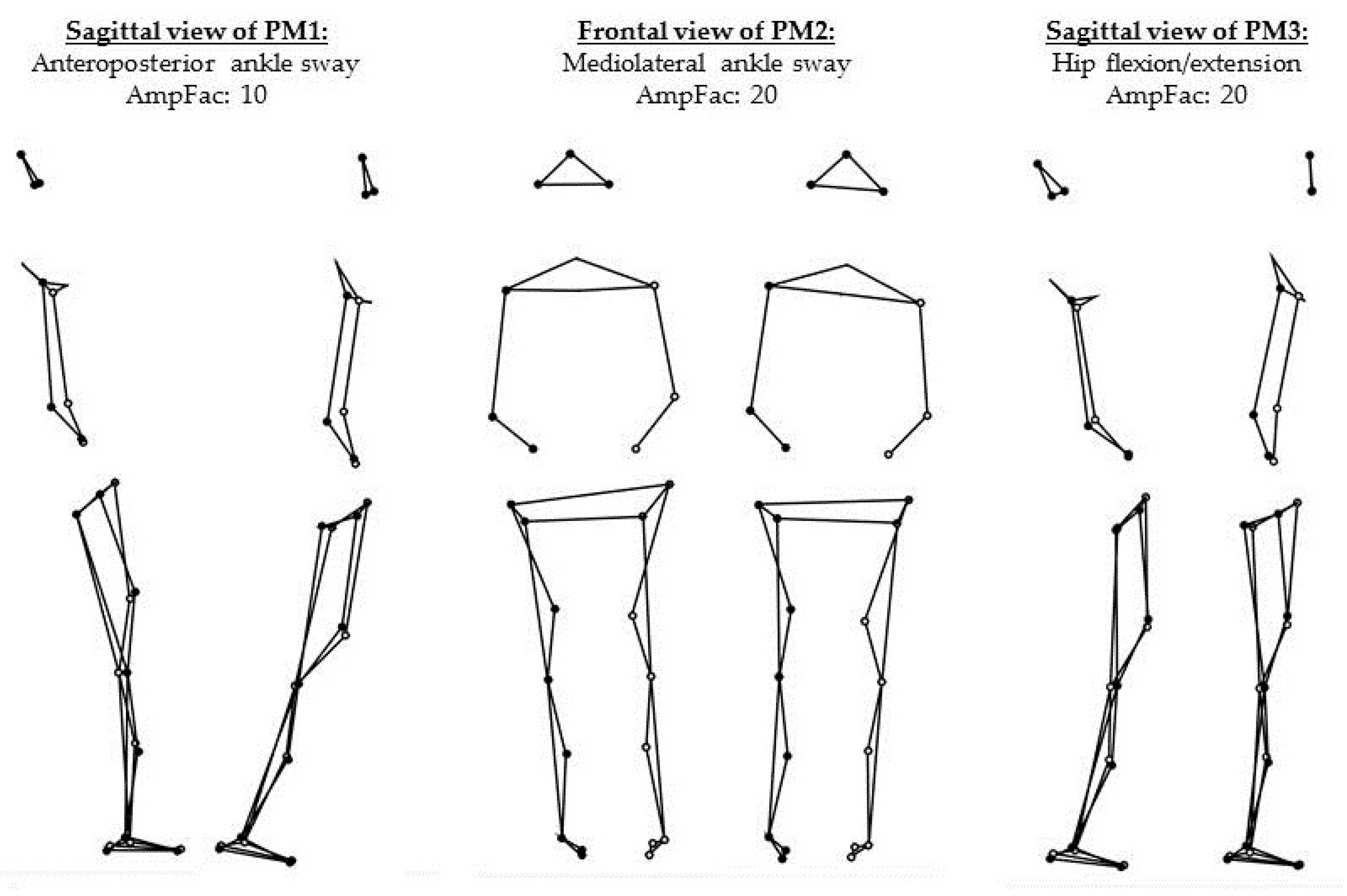
| k | EV (Eigenvalues) (%) | Correlation | Main Strategy (Directions) | Specifications/Additional Features |
|---|---|---|---|---|
| 1 | 77.77 | * | Ankle sway (anterior/posterior) | No visible motions in the rest of the body. |
| 2 | 9.71 | * | Ankle sway (medial/lateral) | No visible motions in the rest of the body. |
| 3 | 4.73 | Hip strategy (flexion/extension) | Resulting upper body sway (posterior/anterior) visible. |
| rSTD1 | rSTD2 | rSTD3 | SaEn (PP1) | SaEn (PP2) | SaEn (PP3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | rcorr | p | rcorr | p | rcorr | p | rcorr | p | rcorr | p | rcorr | |
| COPAP-SaEn | 0.002 | −0.633 | 0.705 | 0.088 | 0.261 | 0.257 | <0.001 | 0.717 | 0.390 | 0.198 | 0.887 | 0.033 |
| COPML-SaEn | 0.150 | 0.326 | <0.001 | −0.753 | 0.565 | 0.133 | 0.384 | 0.200 | <0.001 | 0.893 | 0.730 | 0.080 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haid, T.; Federolf, P. Human Postural Control: Assessment of Two Alternative Interpretations of Center of Pressure Sample Entropy through a Principal Component Factorization of Whole-Body Kinematics. Entropy 2018, 20, 30. https://doi.org/10.3390/e20010030
Haid T, Federolf P. Human Postural Control: Assessment of Two Alternative Interpretations of Center of Pressure Sample Entropy through a Principal Component Factorization of Whole-Body Kinematics. Entropy. 2018; 20(1):30. https://doi.org/10.3390/e20010030
Chicago/Turabian StyleHaid, Thomas, and Peter Federolf. 2018. "Human Postural Control: Assessment of Two Alternative Interpretations of Center of Pressure Sample Entropy through a Principal Component Factorization of Whole-Body Kinematics" Entropy 20, no. 1: 30. https://doi.org/10.3390/e20010030





