A New Thermodynamics from Nuclei to Stars
Abstract
:1 Introduction
- thermodynamics addresses large homogeneous systems at equilibrium (in the thermodynamic limit N → ∞|N/V = ρ,homogeneous).
- Phase transitions are the positive zeros of the grand-canonical partition sum Z(T, µ, V ) as function of eβµ (Yang-Lee-singularities). As the partition sum for a finite number of particles is always positive, zeros can only exist in the thermodynamic limit V|β,µ → ∞.
- Micro and canonical ensembles are equivalent. 1
- thermodynamics works with intensive variables T, P, µ.
- Unique Legendre mapping T → E.
- Heat only flows from hot to cold (Clausius)
- Second law only in infinite systems when the Poincarré recurrence time becomes infinite (much larger than the age of the universe (Boltzmann)).
2 Boltzmann’s principle

3 Topological properties of S(E, ⋯)
3.1 Unambiguous signal of phase transitions in a ”Small” system [5]
3.2 Systematic of phase transitions in the micro-canonical ensemble without invoking the thermodynamic limit
- A single stable phase of course with some intrinsic fluctuations (width) by a negative largest curvature λ1 < 0. Here s(e, n) is concave (downwards bending) in both directions. Then there is a one to one mapping of the canonical ↔the micro-ensemble.
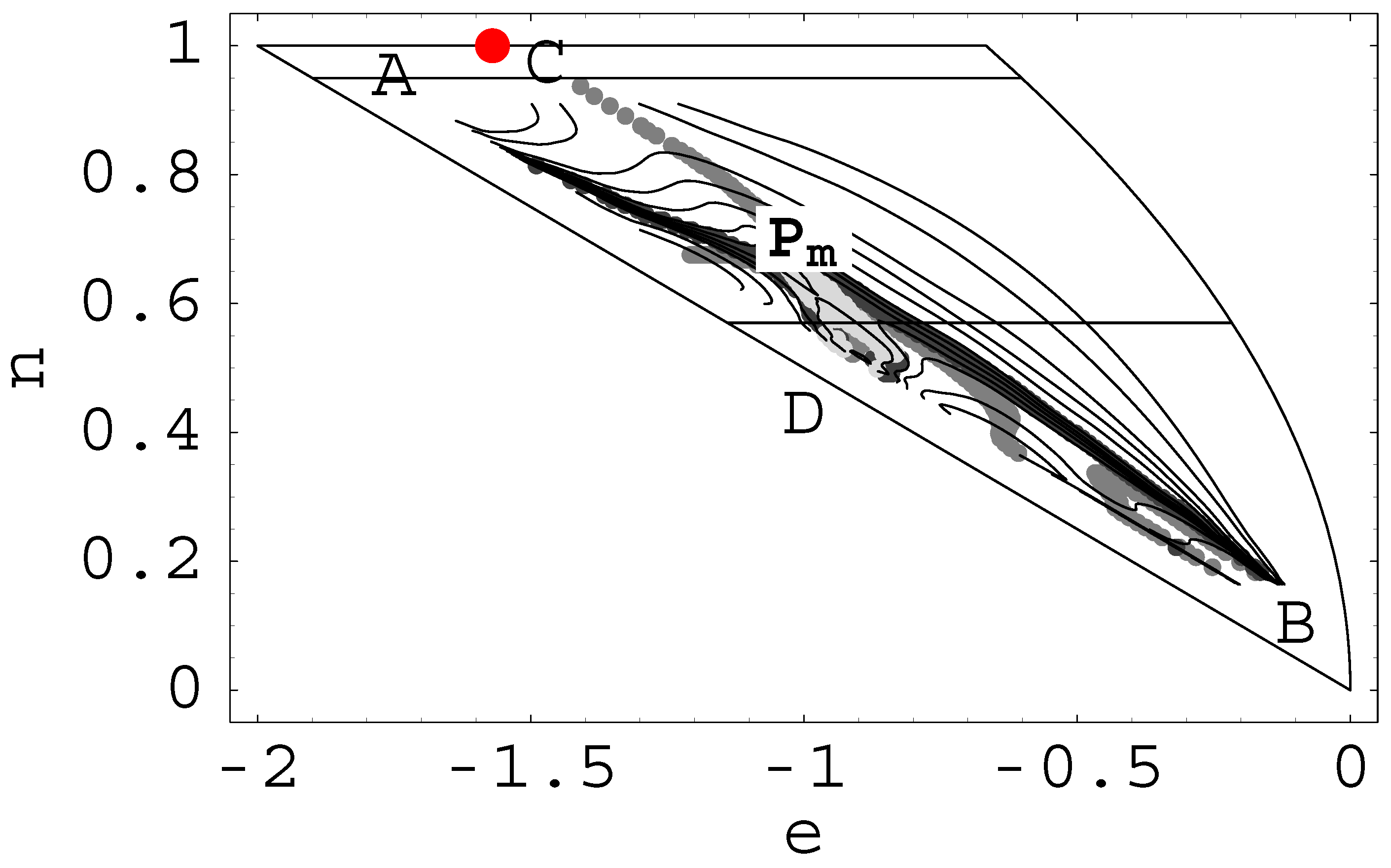
- A transition of first order with phase separation and surface tension is indicated by λ1(e, n) > 0. s(e, n) has a convex intruder (upwards bending) in the direction v1 of the largest curvature ≥ 0 which can be identified with the order parameter [7]. Three solutions ofdetermine the intensive temperature T = 1/β and the chemical potential Tν.In the thermodynamic limit the whole region {o1, o3} is mapped into a single point in the canonical ensemble which is consequently non-local in o. I.e. if the curvature of S(E, N ) is λ1 ≥ 0 both ensembles are not equivalent even in the limit.
- A continuous (“second order”) transition with vanishing surface tension, where two neighboring phases become indistinguishable. This is indicated in figure (1) by a line with λ1 = 0 and extremum of λ1 in the direction of order parameter vλ=0 · ∇λ1 = 0. These are the catastrophes of the Laplace transform E → T .
3.3 CURVATURE
| N0 | 200 | 1000 | 3000 | bulk | |
| Na | Ttr [K] | 940 | 990 | 1095 | 1156 |
| qlat [eV ] | 0.82 | 0.91 | 0.94 | 0.923 | |
| sboil | 10.1 | 10.7 | 9.9 | 9.267 | |
| ∆ssurf | 0.55 | 0.56 | 0.44 | ||
| Nsurf | 39.94 | 98.53 | 186.6 | ∞ | |
| σ/Ttr | 2.75 | 5.68 | 7.07 | 7.41 |
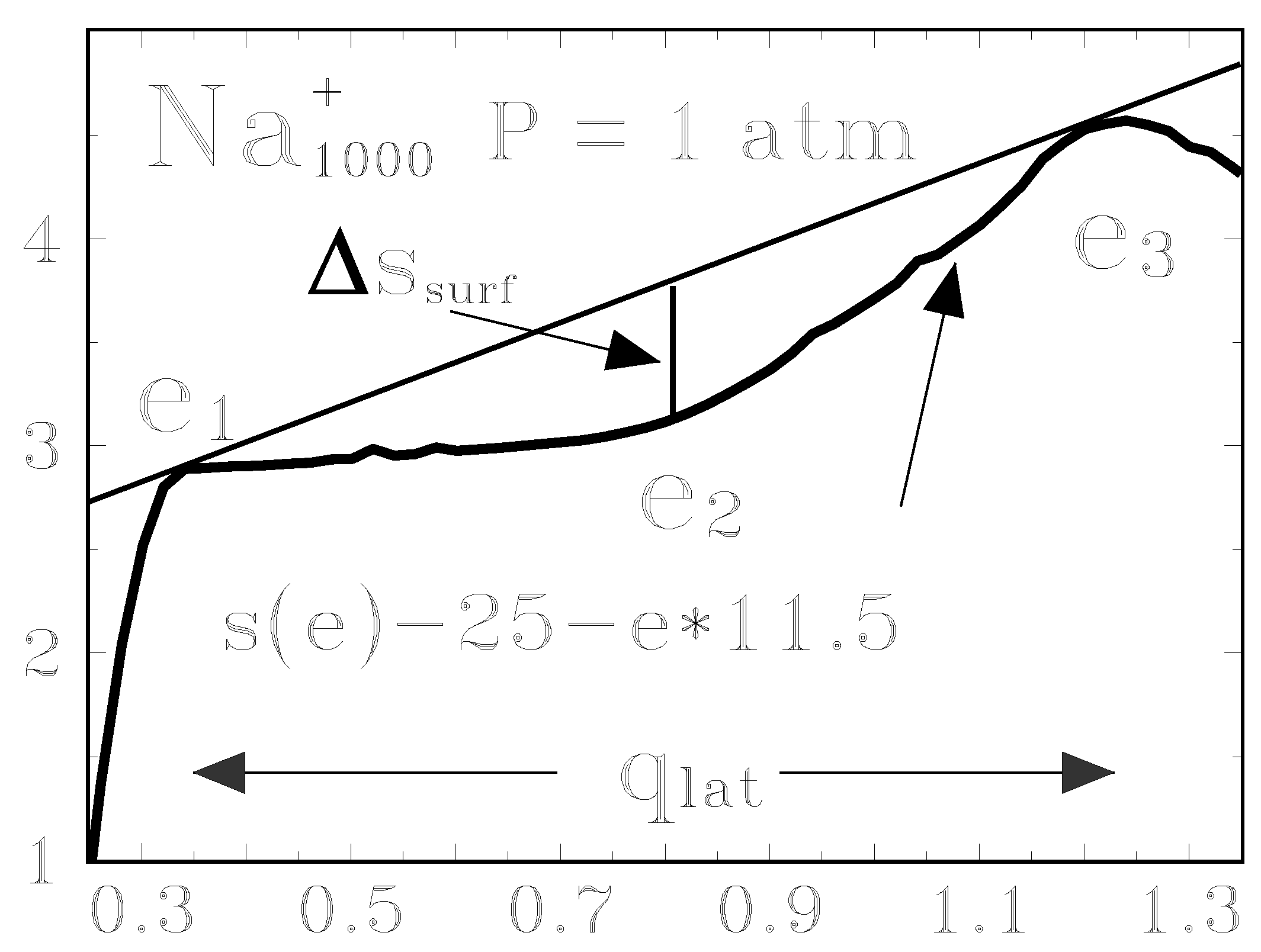
3.4 Heat can flow from cold to hot
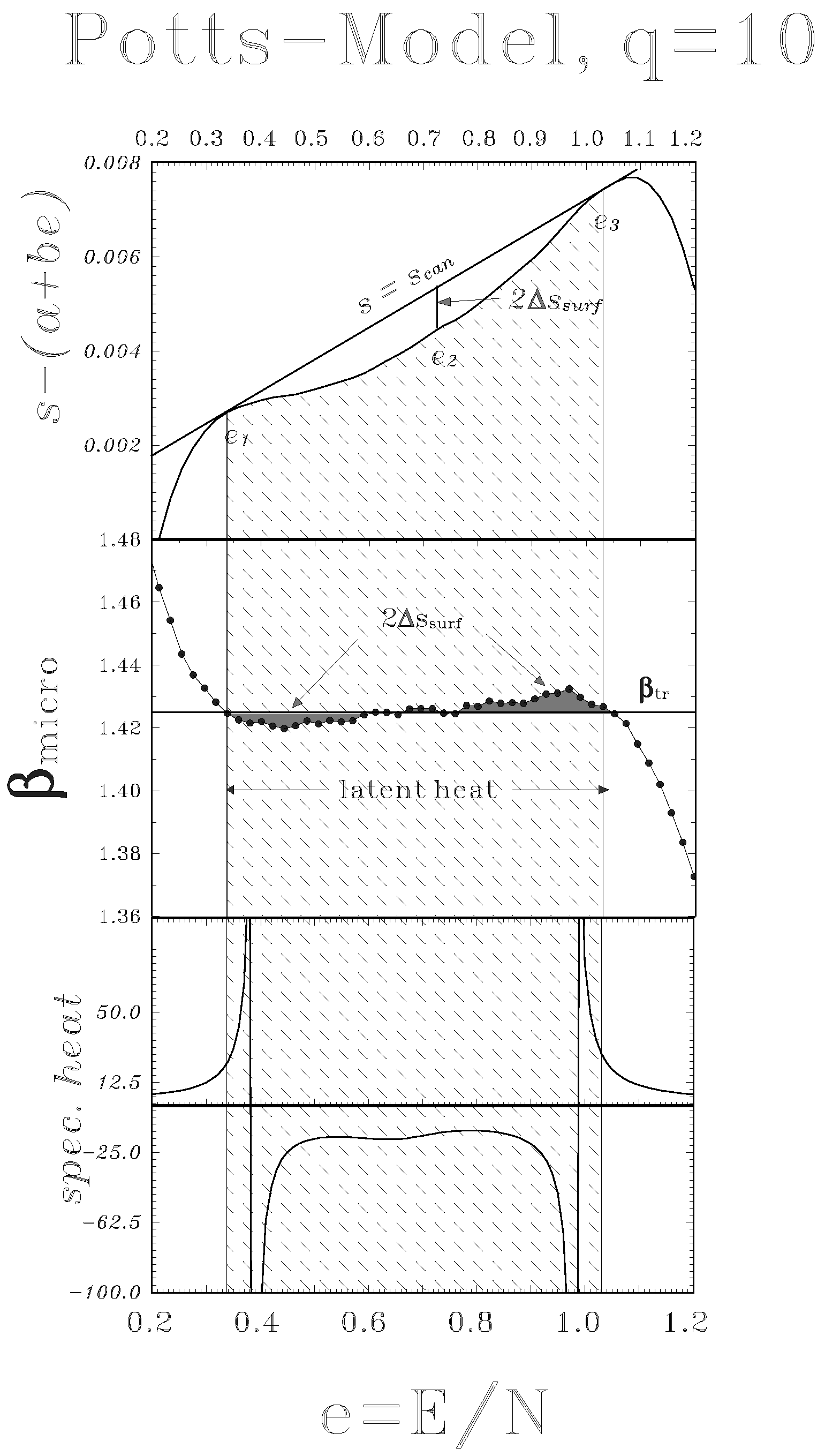
4 Negative heat capacity as signal for a phase transition of first order.
4.1 Nuclear Physics
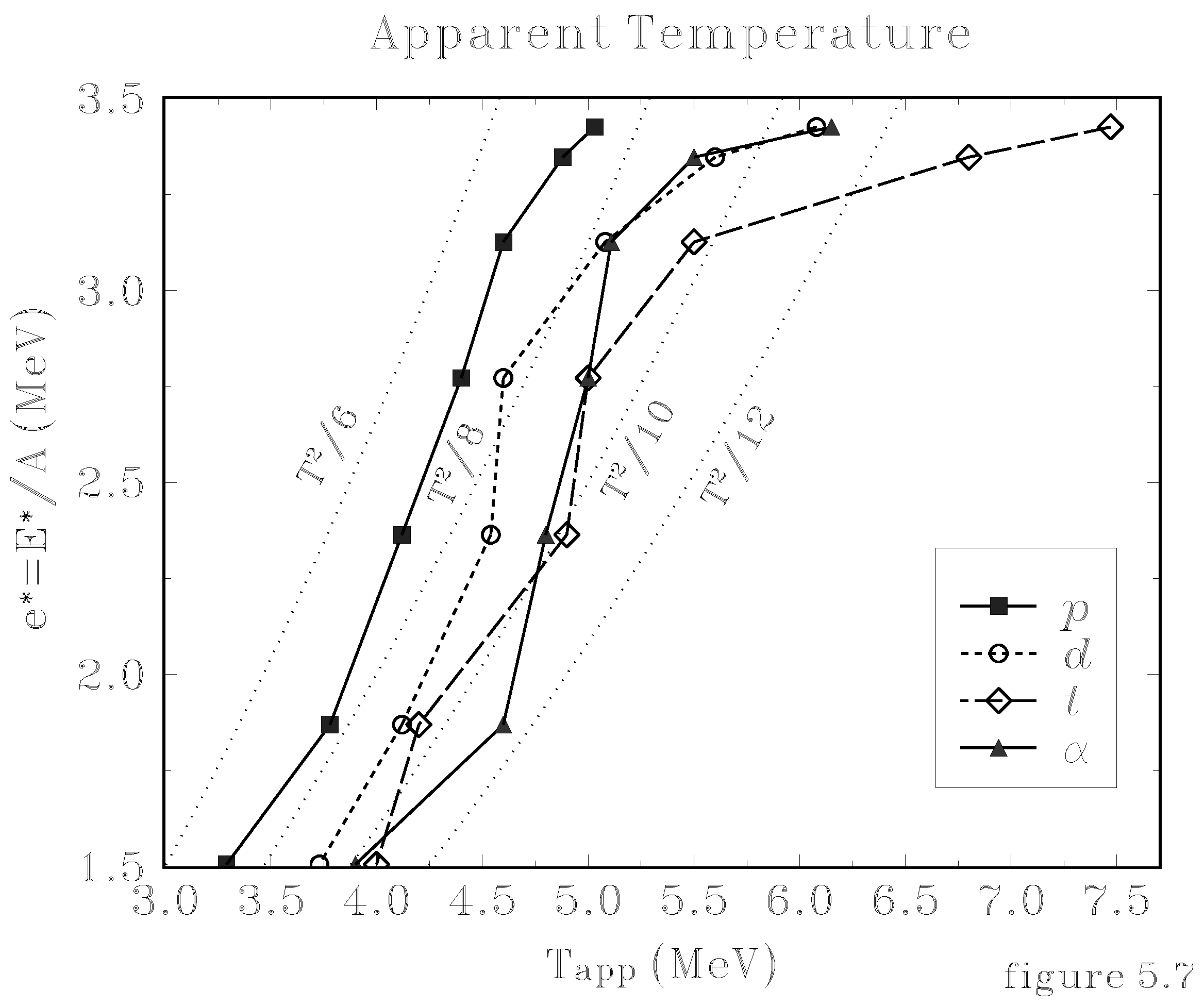
4.2 Atomic clusters

4.3 Stars
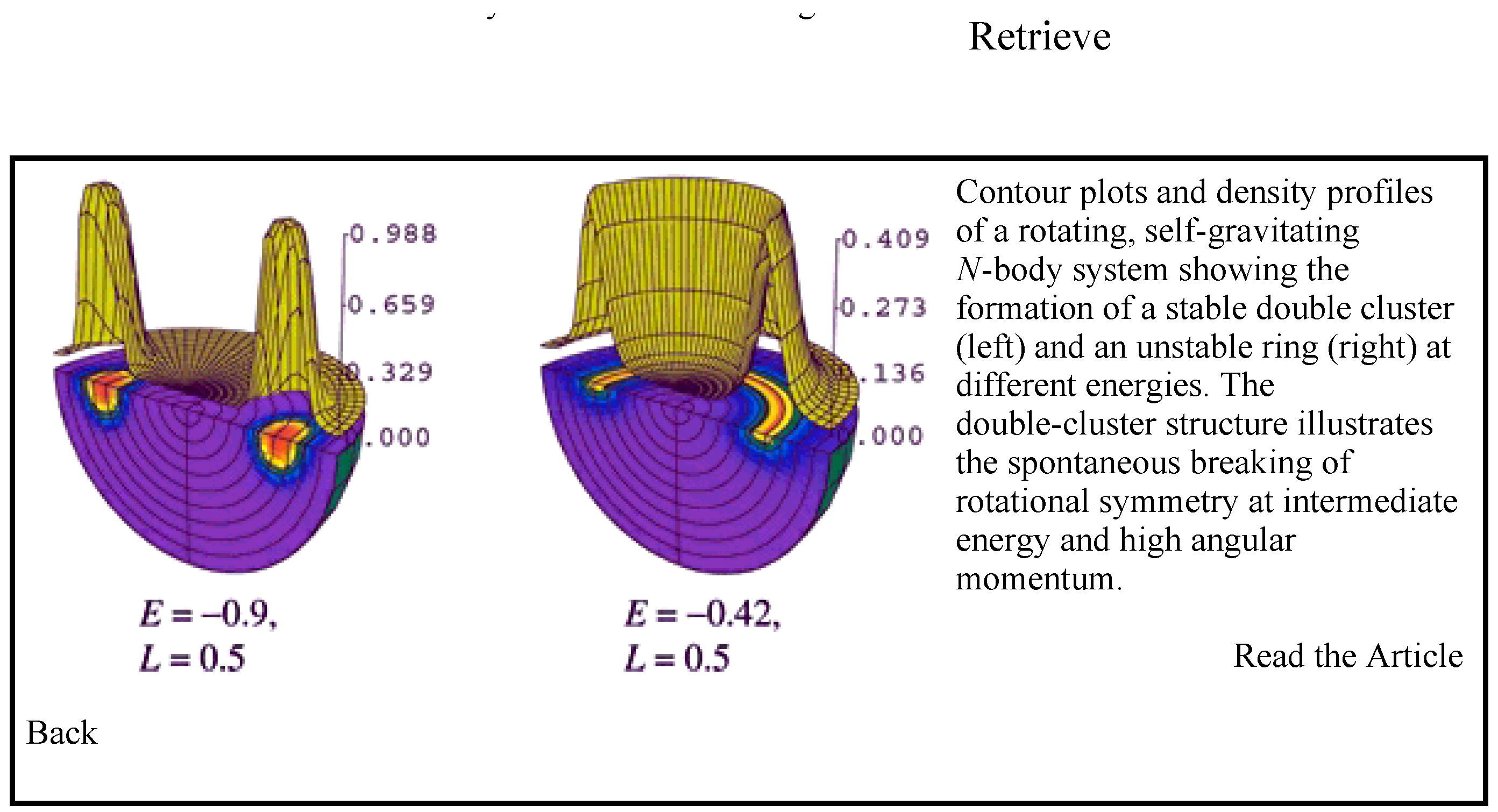
5 The second law, microcanonically
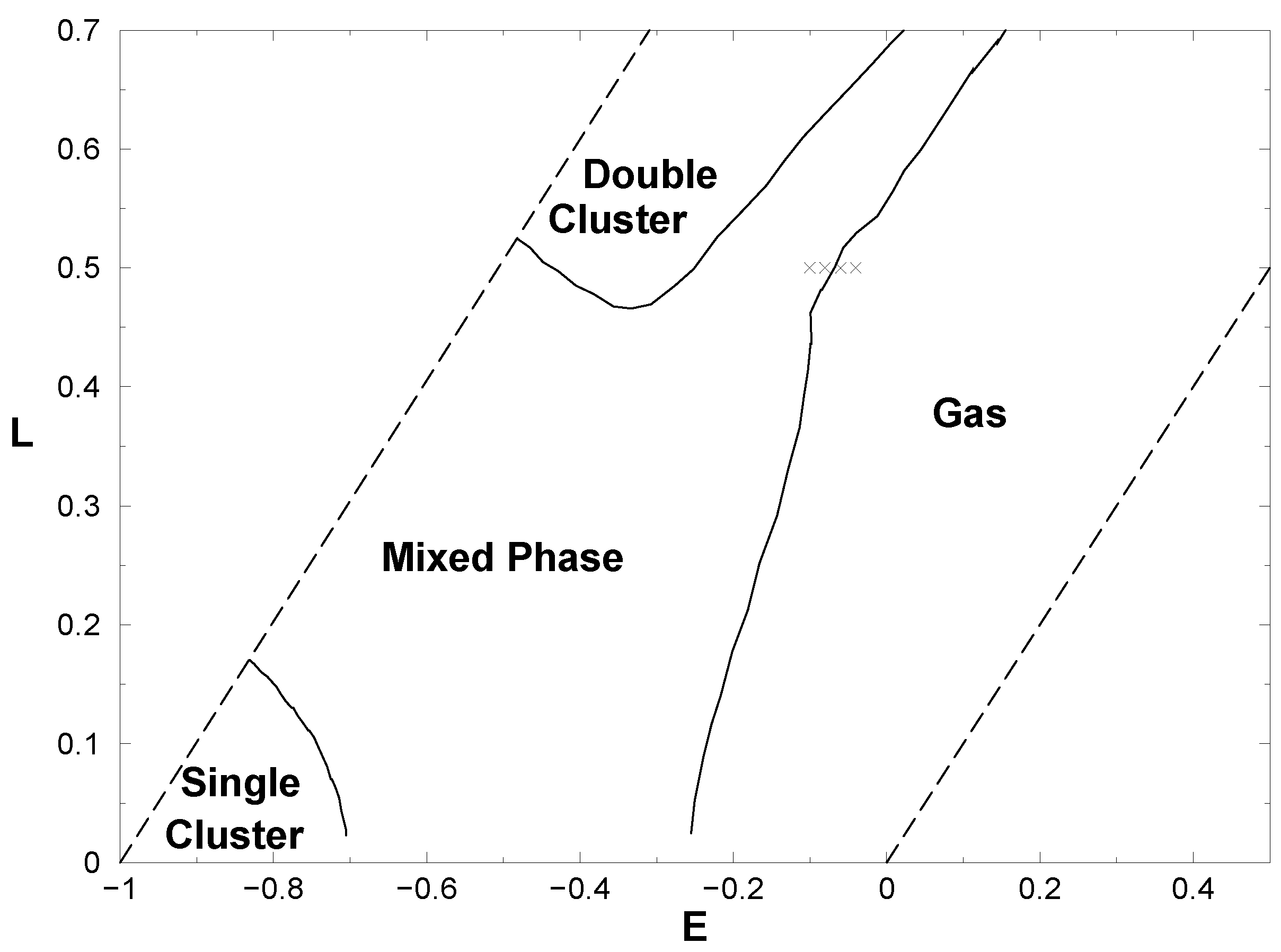
5.1 Measuring a macroscopic observable, the “ EPS-formulation ”
5.2 Fractal distributions in phase space, second law
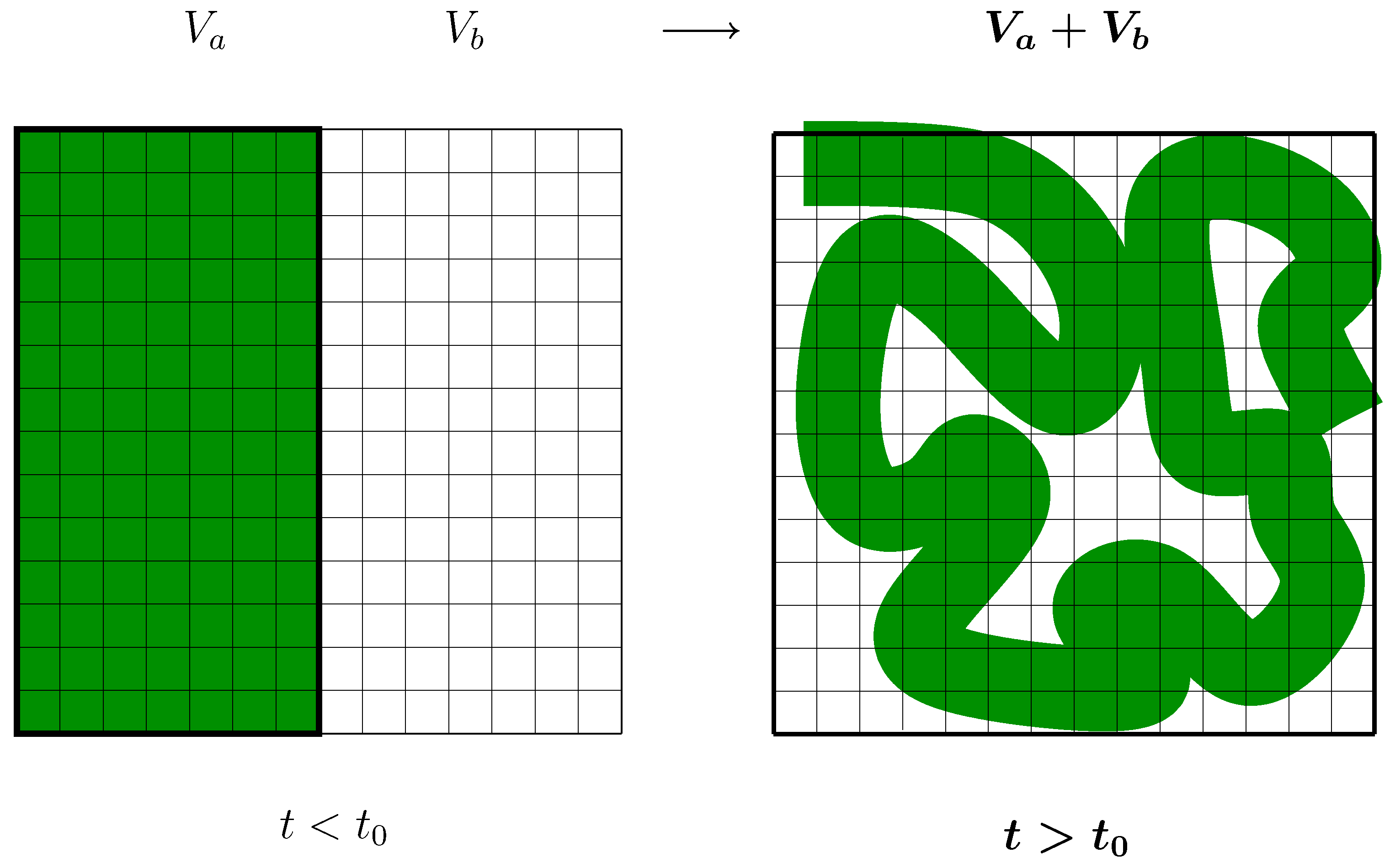
5.3 Conclusion of the discussion of the second law
6 Final conclusion
- at phase-separation (→heat engines !), one gets inhomgeneities, and a negative heat ca-pacity or some other negative susceptibility,
- Heat can flow from cold to hot.
- phase transitions can be localized sharply and unambiguously in small classical or quantum systems, there is no need for finite size scaling to identify the transition.
- also really large self-gravitating systems can now be addressed.
7 Appendix
References
- Goldstein, S.; Lebowitz, J.L. On the (Boltzmann) entropy of nonequilibrium systems. 2003; arXiv:cond-mat/0304251. [Google Scholar]
- Gross, D.H.E. Ensemble probabilistic equilibrium and non-equilibrium thermodynamics without the thermodynamic limit. In Foundations of Probability and Physics; number XIII in PQ-QP: Quantum Probability, White Noise Analysis; Khrennikov, Andrei, Ed.; ACM, World Scientific: Boston, October 2001; pp. 131–146. [Google Scholar]
- Gross, D.H.E. Second law in classical non-extensive systems. In Proceedingsof the First International Conference on Quantum Limits to the Second Law, University of San Diego, 2002; Sheehan, D., Ed.; p. cond{mat/0209467.
- Schrödinger, E. Statistical Thermodynamics, a Course of Seminar Lectures, delivered in January-March 1944 at the School of Theoretical Physics; Cambridge University Press: London, 1946. [Google Scholar]
- Gross, D.H.E.; Votyakov, E. Phase transitions in finite systems = topological peculiarities of the microcanonical entropy surface. 1999, p. 4. http://xxx.lanl.gov/abs/cond-mat/9904073.
- Gross, D.H.E.; Votyakov, E. Phase transitions in ”Small” systems. Eur.Phys.J.B 2000, 15, 115–126. http://arXiv.org/abs/cond-mat/?9911257. [Google Scholar]
- Gross, D.H.E. MicrMicrocanonical thermodynamics: Phase transitions in “Small” systems, volume 66 of Lecture Notes in Physics, World Scientific: Singapore, 2001.
- Bréchignac, C.; Cahuzac, Ph.; Carlier, F.; Leygnier, J.; Roux, J.Ph. J.Chem.Phys. 1995, 102, 1.
- D’Agostino, M.; Gulminelli, F.; Chomaz, Ph.; Bruno, M.; Cannata, F.; Bougault, R.; Gramegna, F.; Iori, I.; le Neindre, N.; Margagliotti, G.V.; Moroni, A.; Vannini, G. Negative heat capacity in the critical region of nuclear fragmentation: an experimental evidence of the liquid-gas phase transition. Phys.Lett.B 2000, 473, 219–225. [Google Scholar]
- Einstein, A. Kinetische Theorie des Wärmegleichgewichtes und des zweiten Hauptsatzes der Thermodynamik. Annalen der Physik 1902, 9, 417–433. [Google Scholar]
- Einstein, A. Eine Theorie der Grundlagen der Thermodynamik. Annalender Physik 1903, 11, 170–187. [Google Scholar]
- Lieb, Elliott H.; Yngvason, J. The physics and mathematics of the second law of thermodynamics. Physics Report,cond-mat/9708200 1999, 310, 1–96. [Google Scholar]
- Lieb, E.H.; Yngvason, J. A guide to entropy and the second law of thermodynamics. mat-ph/9805005, 1998. [Google Scholar]
- Lebowitz, J.L. Microscopic origins of irreversible macroscopic behavior. Physica A 1999, 263, 1–527. [Google Scholar]
- Lebowitz, J.L. Statistical mechanics: A selective review of two central issues. Rev.Mod.Phys. 1999, 71, S346–S357. [Google Scholar]
- Krylov, N.S. Works on the Foundation of Statistical Physics; Princeton University Press: Princeton, 1979. [Google Scholar]
- Fox, R.F. Entropy evolution for the baker map. Chaos 1998, 8, 462–465. [Google Scholar]
- Gilbert, T.; Dorfman, J.R.; Gaspard, P. Entropy production. Phys.Rev.Lett. 2000, 85, 1606, nlin.CD/0003012. [Google Scholar]
- Goldstein, H. Classical Mechanics; Addison-Wesley, Reading, Mass, 1959. [Google Scholar]
- Falconer, Kenneth. Fractal Geometry - Mathematical Foundations and Applications; John Wiley & Sons: Chichester, New York, Brisbane, Toronto,Singapore, 1990. [Google Scholar]
- Weisstein, E.W. Concise Encyclopedia of Mathemetics; CRC Press: London, New York, Washington D.C, 1999. [Google Scholar]
- Loschmidt, J. Wienerberichte 1876, 73, 128.
- Zermelo, E. Wied. Ann. 1896, 57, 778–784.
- Zermelo, E. Über die mechanische Erklärung irreversibler Vorgänge. Wied.Ann. 1897, 60, 392–398. [Google Scholar]
- Cohen, E.G.D. Boltzmann and statistical mechanics. In Boltzmann’s Legacy, 150 Years after his Birth. pages http://xxx.lanl.gov/abs/cond–mat/9608054Rome, 1997; Atti dell Accademia dei Lincei. [Google Scholar]
- Cohen, E.G.D. Boltzmann and Statistical Mechanics, volume 371 of Dynamics: Models and Kinetic Methods for Nonequilibrium Many Body SystemsKluwer: Dordrecht, The Netherlands, e, j. karkheck edition; 2000. [Google Scholar]
- 1How does one normally prove this?: The general link between the microcanonical probability eS(E,N) and the grand-canonical partition function Z(T, µ) for extensive systems is by the Laplace transform:If s(e, n) is concave then there is a single point es, ns withIn the thermodynamic limit (V → ∞) the quadratic approximation in equ.(3) becomes exact and there is a one to one mapping of the microscopic mechanical e = E/V, n = N/V to the intensive T, µ.
- 2In this paper we denote ensembles or manifold in phase space by calligraphic letters like
©2004 by MDPI (http://www.mdpi.org). Reproduction for noncommercial purposes permitted.
Share and Cite
Gross, D.H.E. A New Thermodynamics from Nuclei to Stars. Entropy 2004, 6, 158-179. https://doi.org/10.3390/e6010158
Gross DHE. A New Thermodynamics from Nuclei to Stars. Entropy. 2004; 6(1):158-179. https://doi.org/10.3390/e6010158
Chicago/Turabian StyleGross, Dieter H.E. 2004. "A New Thermodynamics from Nuclei to Stars" Entropy 6, no. 1: 158-179. https://doi.org/10.3390/e6010158






