Introduction
Maxwell [
1] drew attention to the possibility that the second law could be limited in its application by introducing a “neat-fingered being”, later to be called a demon, to control a flow of gas molecules and create a chemical potential gradient at the expense of heat. However, Maxwell finally dismissed the idea that the control was to have intelligence and insisted that it should be viewed as a valve [
2]. This conjecture remains with its original force because to provide a refutation, an examination must be made of
all relevant processes and structures and a proof showing that the examination is complete. To discard the concept of the Maxwellian valve is no mere task and often such attempts are either circular [
3] or are too specific [
4].
Nature has provided many examples of membrane carriers where the major function is the creation of gradients of chemical potential across cell walls. One such example is known as the sodium-potassium ATPase, an enzyme that carries sodium ions out of the cell and potassium ions into the cell and the mechanism is driven by the hydrolysis of ATP. We can draw on models of the ATPase to make a theoretical construct of an enzyme that acts as a Maxwellian valve. To accomplish this, the chemical energy of the hydrolysis is replaced by thermal energy from a single heat reservoir.
The system (
Figure 1) of a Maxwellian valve has been described in a preliminary sketch [
5]. It concerns the flux of a solute (S) through a heat conducting membrane via an embedded enzyme molecule that rotates around an axis (x) normal to the membrane surface. The solute is adsorbed onto the enzyme from either one of the solutions bathing the membrane and after a conformational change of the enzyme, the solute is released to the other solution. The two solutions have the same temperature. The adsorbed solute can traverse the membrane in both directions and the nature of the adsorption bond remains constant and independent of location of the adsorption site. However, the rotation of the enzyme in one of its configurations (Q) affects the free energy of adsorption and it will be shown that this leads to a net unidirectional flux of the solute. The flow occurs even in the absence of a gradient of chemical potential between the solutions and is thereby a mechanism for perpetual motion of the second kind. Onsager [
6] saw the possibility of microscopic irreversibility within conservative systems where mechanical forces such as centrifugal, Coriolis and external magnetic forces play an important role. That is, in situations where the forces are odd functions of the velocity.
Thus, the total system to be analysed can be described briefly as follows. Two solutions of a solute S are separated by a membrane that contains an enzyme. The enzyme exists in two conformations, P and Q, both of which can adsorb a molecule of solute to become SP and SQ respectively.
Also conformational transitions exist between P and Q and between SP and SQ.
The four reactions form a cycle (
Fig. 1) in which a solute molecule is transferred from one solution to the other. We shall assume, as is normal in chemical kinetics, that each reaction is independent of the past history of the process. That is, the available events occur randomly. A large number of events may occur before one cycle is completed and cycles can be clockwise or anticlockwise.
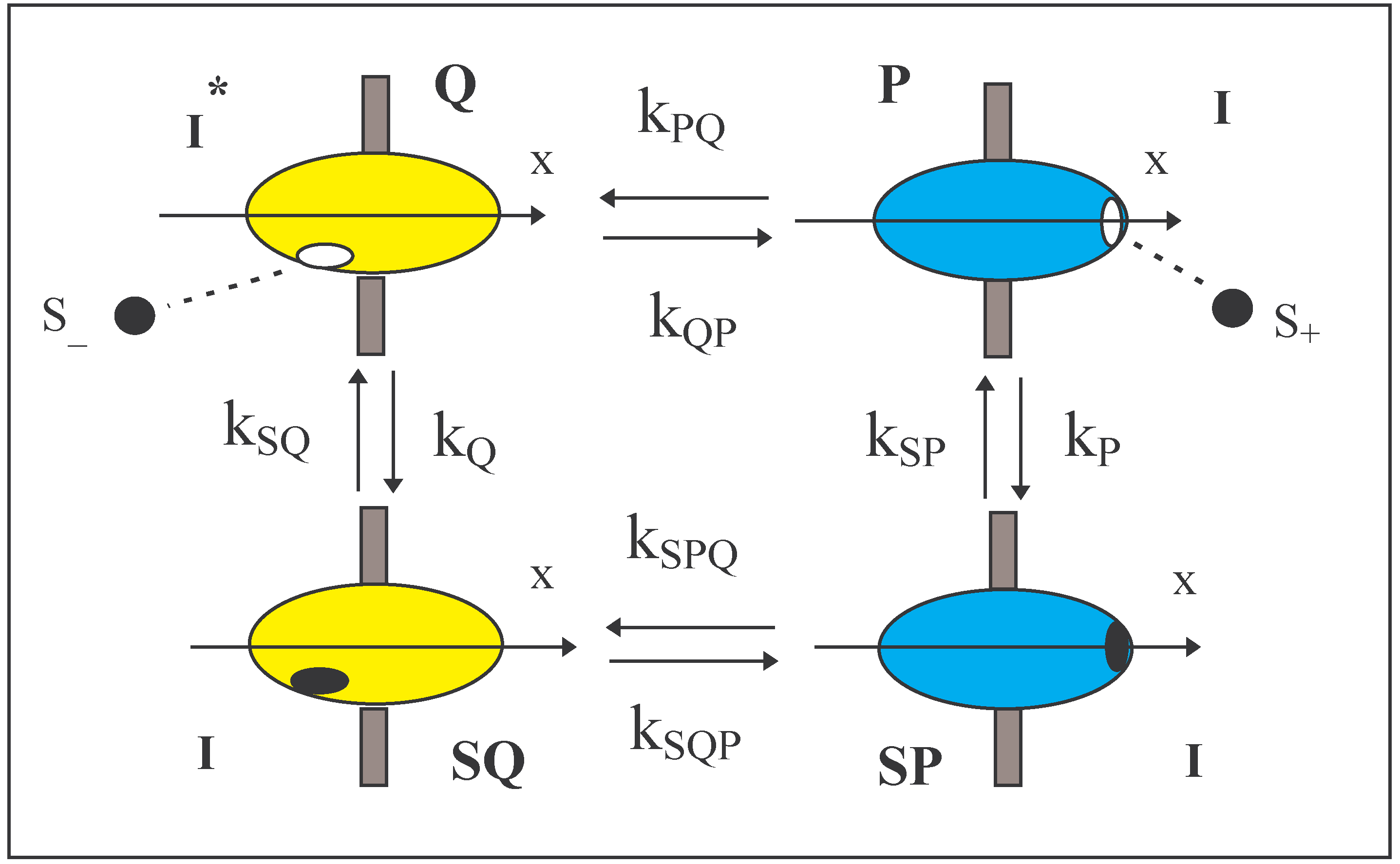
FIGURE 1.
Four states of an enzyme are shown. Two of the states, P and Q, are conformers of the enzyme and the two other states are the same conformers SP and SQ respectively on which a solute molecule of S is adsorbed. The enzyme is embedded in a membrane with its x-axis perpendicular to the membrane. The adsorption sites on P and SP lie on one side of the membrane and those of Q and SQ lie on the other. The sites, occupied or empty, pass through the membrane intact during conformational changes. Q has a different moment of inertia from the other states.
FIGURE 1.
Four states of an enzyme are shown. Two of the states, P and Q, are conformers of the enzyme and the two other states are the same conformers SP and SQ respectively on which a solute molecule of S is adsorbed. The enzyme is embedded in a membrane with its x-axis perpendicular to the membrane. The adsorption sites on P and SP lie on one side of the membrane and those of Q and SQ lie on the other. The sites, occupied or empty, pass through the membrane intact during conformational changes. Q has a different moment of inertia from the other states.
Structures
The enzyme (
Fig. 1) has an axis of rotation defined as the x-axis and the y-z plane passes through the enzyme and is orthogonal to the x-axis. Furthermore, the membrane lies in the y-z plane. The locations of the adsorption site play an important role in the design. In all states, the adsorption site is in the surface of the enzyme. On P and PS the adsorption site lies on the x-axis at a positive value of x. On Q and QS the adsorption site is on the opposite side at a negative value of x and lies at a distance ‘
r’ from the axis. [S
+] and [S
-] are the concentrations of S in the two bathing solutions with their subscripts indicating their locations according to x.
Moments of Inertia
In each of the four reaction pathways in the cycle, there is an associated change in free energy and this can be divided into chemical and physical components, the latter being the contribution from the energy of rotation around the x-axis. As the argument presented here does not require complete generality, simplifications can be made. We choose to select the condition that three of the structures have the same moment of inertia with reference to the x-axis. SP and SQ can be given the same moment of inertia (= I) since the distribution of mass in the two conformers is not constrained by any condition given above. Now P and SP are identical except for the adsorbate, S. However, S provides no contribution to the moment of inertia of SP since it is attached to P on the axis of rotation. Even if S contained elements far from the axis, its attachment through a single bond directed along the axis would not allow any torque to be expressed between S and P through the bond
1. Thus, P has the same moment of inertia as SP (= I). If the mass of S is ‘m’ then the moment of inertia of SQ differs from the moment of inertia of Q by the amount IS where
Free Energy Differences
In this section, we will assume that the second law is valid for the above system and that the initial conditions can be arranged so that each pair of adjacent states is in equilibrium. A consequence of this assumption is that [S+] and [S-] will be equal and should remain so with time. Also without affecting the argument we can make the simplifying assumption that the free energy difference between P and Q is zero when the two conformers have no angular velocity around the axis of rotation. (The free energies in the absence of rotation depend on the intramolecular bonds of the conformers and the interactions of the conformers with the membrane and the bathing fluids). There is no previously imposed condition that would invalidate this assumption.
Thus, it follows from (1) that in general, the difference in free energy between P and Q is given by the rotational component alone.
where N is Avogadro’s number and
ω is the angular velocity of the Brownian motion.
The equilibrium between P and Q can also be expressed in chemical thermodynamical terms as
where K
PQ is the equilibrium constant, k is the Boltzmann constant and T is the absolute temperature. With the subscripted k as a rate constants, we obtain from the preceding two equations
Since the
chemical nature of the adsorption bond is the same for P and Q, it follows that the free energy difference between SP and SQ is also zero. Furthermore, since the moments of inertia of SP and SQ are identical, their free energy difference is independent of rotation velocity.
Therefore
The standard free energy of adsorption at the adsorption site on P is given by
where K
ads has no rotational component
The standard free energy of adsorption at the adsorption site on Q has a rotational component.
A simple interpretation of equations (2) and (5) draws on the fact that the centrifugal force increases the probabilities of the transformations SQ to Q and Q to P. In the remaining section of the cycle involving SP, the centrifugal force has no influence. This is the foundation of the irreversibility about which Onsager [
6] surmised.
Kinetic Equations
Following in the manner of Onsager [
6], we obtain a net clockwise reaction rate given by
where [Enzyme ] is the total concentration of the enzyme carrier in moles per unit area of membrane and
The four rows of Δ are obtained from the matrix of equations based on the total concentration of enzyme
and on d[P]/dt, d[Q]/dt and d[SP]/dt being zero for the steady state (or the assumed equilibrium).
Onsager, in discussing cyclic processes, drew attention to the thermodynamic importance of expression (6) noting that it would be zero when the processes had reached equilibrium. This of course was a conclusion he reached in his discussion on reversible reactions. Although in the same paper, Onsager introduced some concepts of microscopic irreversibility, he did not discuss these advances with regard to cyclic processes nor did he show how they might have been conceived within the framework of chemical reactions.
By the substitution of the results of equations (2)-(5) in (6) we obtain the net anti-clockwise
flux, J.
All factors of (7) are non-zero when [S
+]=[S
-]. Thus the above system generates from solute equilibrium a non-zero chemical potential gradient which tends to a maximum given by
The resulting gradient is available for work at the expense of the heat of the system.
Because the above scheme develops a net non-zero flux given by (7), the system can not be in equilibrium, which contradicts our assumption. Namely that the second law was valid and that the system could exist at equilibrium with [S+] = [S-]. As the additional simplifying assumptions affect only the complexity of the equations and not the non-equilibrium status of the system, one can only conclude that the universality of the second law has been refuted in this particular case.