Polymeric Particulates to Improve Oral Bioavailability of Peptide Drugs
Abstract
:Introduction
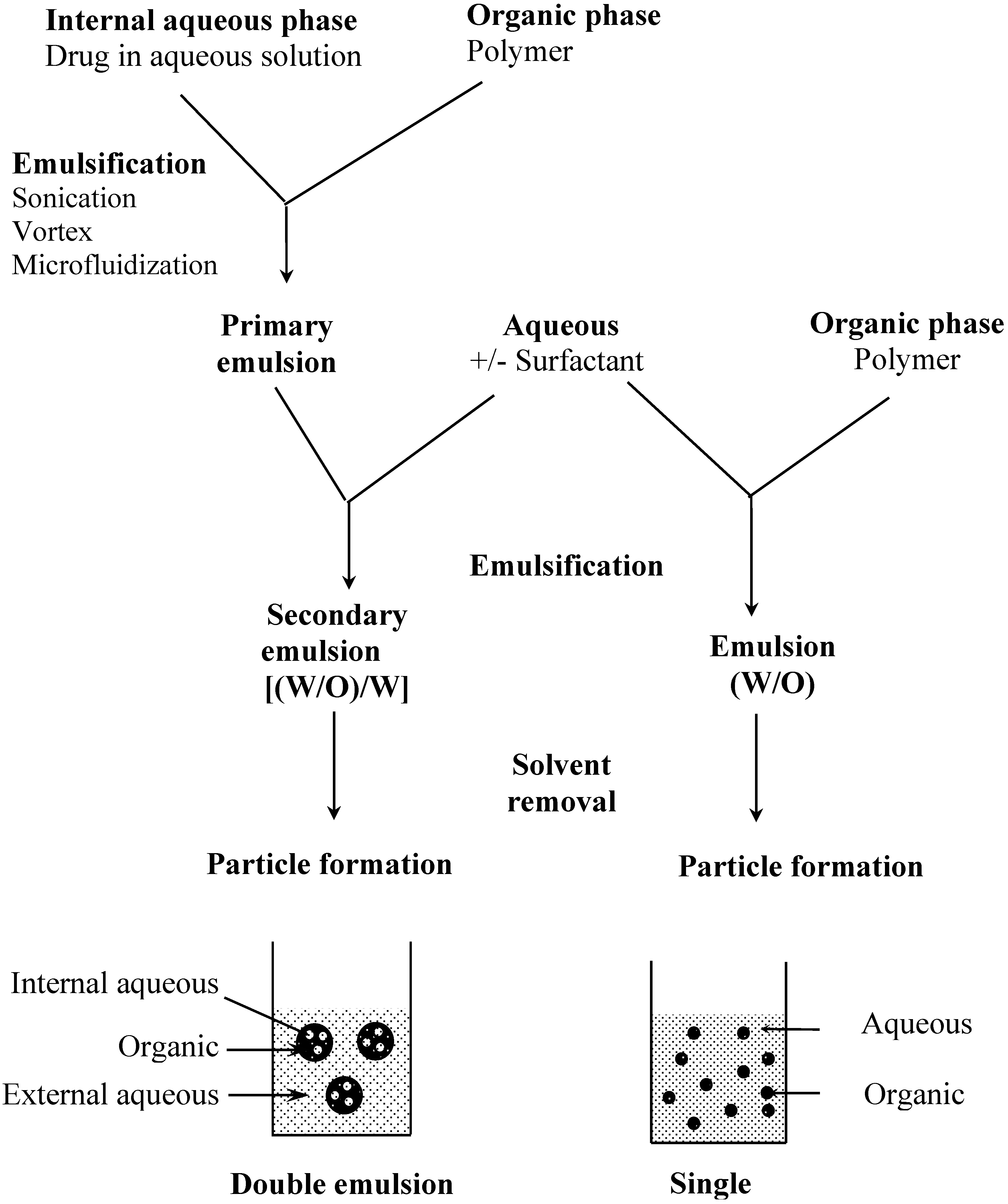
Polymeric Particulates: Definitions and Manufacturing
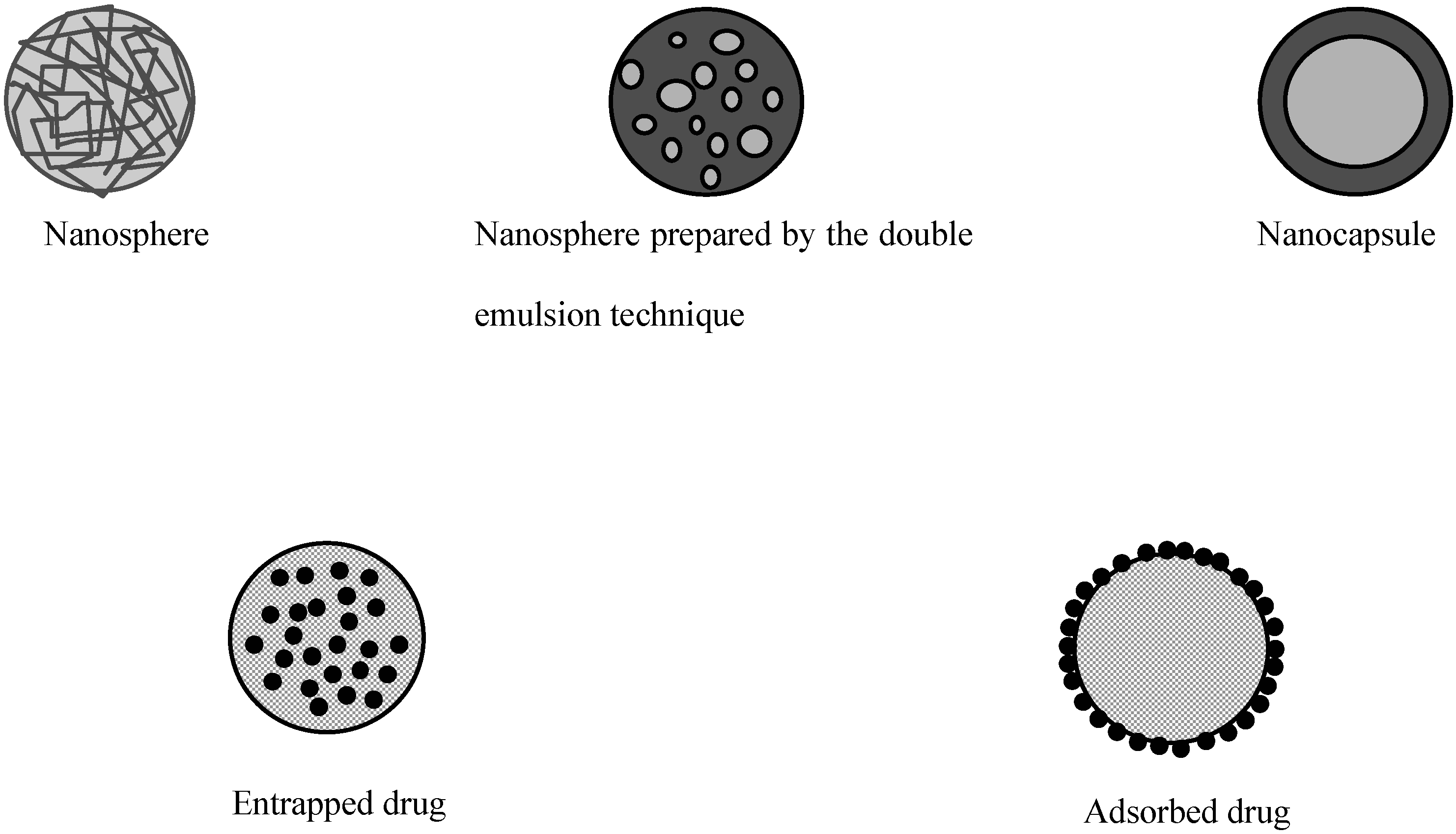
| Polymer | Method | Nature of the particles | References |
|---|---|---|---|
| PLA | Emulsion-evaporation | Spheres | [62] |
| PLGA | Emulsion-evaporation | Spheres | [62] |
| PACA | Interfacial polymerization | Capsules | [67] [24] |
| Chitosan | Emulsion-diffusion | Spheres | [70] |
| Eudragit® | Emulsion-diffusion | Spheres | [71] |
| P(MMA-g-EG) | Suspension polymerization | Spheres | [72] |
| HPMC-AS | Emulsion-evaporation | Spheres | [73] |
| Poly FA:SA | Phase inversion | Spheres | [74] |
| Insulin | Dessolvation-crosslinking | Spheres | [19] |
| LHRH-n-butylcyanoacrylate | Copolymerization | Spheres | [20] |
| Cyclosporin A | Emulsion-precipitation | Spheres | [22] |
| Derivatized amino acids | Self-aggregation | Spheres | [75, 76] |
| Soybean protein hydrolysates | Self-aggregation | Spheres | [77] |
- Key to abbreviations :
- PLA : poly(lactic acid)
- PLGA : poly(lactic-co-glycolide)
- PACA : poly(alkylcyanoacrylate)
- Eudragit® : poly(methylacrylic acid-co-methylmethacrylate)
- P(MAA-g-EG) : poly(methacrylic-g-ethylene glycol)
- FA:SA : fumaric acid:sebacic acid
- HPMC-AS : hydroxypropylmethylcellulose acetate succinate
- LHRH : luteinizing hormone releasing hormone
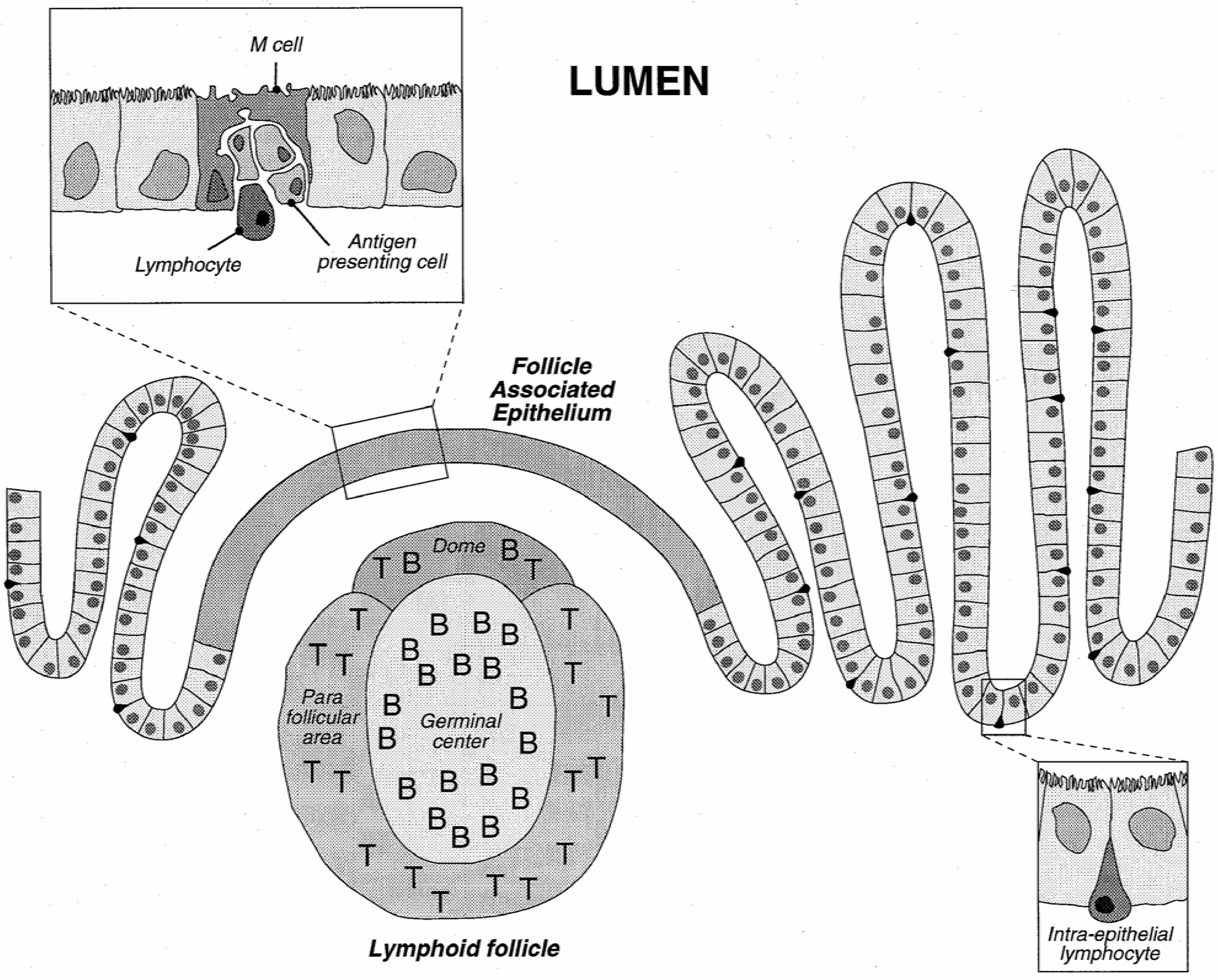
Absorption of Polymeric Particulates from the Gastrointestinal Tract
Polymeric Particles for the Oral Administration of Peptides
Conclusions
References
- Lee, V. H. L.; Robinson, J. R. Enzymatic Barriers to Peptide and Protein Absorption. CRC Crit. Rev. Ther. Drug Carrier Syst. 1988, 5, 69–97. [Google Scholar]
- Allémann, E.; Leroux, J. C.; Gurny, R. Polymeric Nano- and Microparticles for the Oral Delivery of Peptides and Peptidomimetics. Adv. Drug Del. Rev. 1998, 34, 171–189. [Google Scholar]
- Allémann, E.; Gurny, R.; Doelker, E. Drug Loaded Nanoparticles - Preparation Methods and Drug Targeting Issues. Eur. J. Pharm. Biopharm. 1993, 39, 173–191. [Google Scholar]
- Alonso, M. J. Nanoparticulate Drug Carrier Technology. Drugs Pharm. Sci. 1996, 77, 203–242. [Google Scholar]
- Couvreur, P.; Dubernet, C.; Puisieux, F. Controlled Drug Delivery With Nanoparticles: Current Possibilities and Future Trends. Eur. J. Pharm. Biopharm. 1995, 41, 2–13. [Google Scholar]
- Soppimath, K. S.; Aminabhavi, T. M.; Kulkarni, A. R.; Rudzinski, W. E. Biodegradable Polymeric Nanoparticles As Drug Delivery Devices. J. Control. Release 2001, 70, 1–20. [Google Scholar]
- Couvreur, P.; Kante, B.; Roland, M.; Guiot, P.; Bauduin, P.; Speiser, P. Polycyanoacrylate Nanocapsules As Potential Lysosomotropic Carriers: Preparation, Morphological and Sorptive Properties. J. Pharm. Pharmacol. 1979, 31, 331–332. [Google Scholar] [CrossRef]
- Kreuter, J.; Speiser, P. P. In Vitro Studies of Poly(Methyl Methacrylate) Adjuvants. J. Pharm. Sci. 1976, 11, 1624–1626. [Google Scholar]
- Lescure, F.; Seguin, C.; Breton, P.; Bourrinet, P.; Roy, D.; Couvreur, P. Preparation and Characterization of Novel Poly(Methylidene Malonate 2.1.2.)-Made Nanoparticles. Pharm. Res 1994, 11, 1270–1277. [Google Scholar]
- Berton, M.; Benimetskaya, L.; Allémann, E.; Stein, C. A.; Gurny, R. Uptake of Oligonucleotide-Loaded Nanoparticles in Prostatic Cancer Cells and Their Intracellular Localization. Eur. J. Pharm. Biopharm. 1999, 47, 119–123. [Google Scholar]
- Zimmer, A. Antisense Oligonucleotide Delivery With Polyhexylcyanoacrylate Nanoparticles As Carriers. Methods 1999, 18, 286–295. [Google Scholar]
- Blanco-Prieto, M. J.; Delie, F.; Fattal, E.; Tartar, A.; Puisieux, F.; Gulik, A.; Couvreur, P. Characterization of V3BRU Peptide Loaded Small PLGA Microspheres Prepared by (W1/O)W2 Emulsion Solvent Evaporation. Int. J. Pharm. 1994, 111, 137–145. [Google Scholar] [CrossRef]
- Rafati, H.; Coombes, A. G. A.; Adler, J.; Holland, J.; Davis, S. S. Protein-Loaded Poly(DL-Lactide-Co-Glycolide) Microparticles for Oral Administration: Formulation, Structural and Release Characteristics. J. Contr. Rel. 1997, 43, 89–102. [Google Scholar]
- Delie, F.; Berton, M.; Allémann, E.; Gurny, R. Comparison of Two Methods of Encapsulation of an Oligonucleotide into Poly(D,L-Lactic Acid) Particles. Int. J. Pharm. 2001, 214, 25–30. [Google Scholar]
- Bruhn, B. W.; Müller, B. W. Preparation and Characterization of Spray-Dried Poly(Dl-Lactide) Microspheres. Proceed. Int. Symp. Contr. Rel. Bioact. Mater. 1991, 18, 668–669. [Google Scholar]
- Kissel, T.; Brich, Z.; Bantle, S.; Lankranjan, J.; Nimmerfall, F.; Vit, P. Parenteral Depot-Systems on the Basis of Biodegradable Polyesters. J. Contr. Rel. 1991, 16, 27–42. [Google Scholar]
- Blanco-Príeto, M. J.; Orsolini, P.; Heimgartner, F.; Besseghir, K.; Merkle, H. P.; Gander., B. Importance of the Test Medium for the Release Kinetics of a Somatostatin Analogue From Poly(D,L-Lactide-Co-Glycolide) Micropheres. Int. J. Pharm. 1999, 184, 243–250. [Google Scholar]
- Gupta, P. K.; Johnson, H.; Alexon, C. In Vitro and in Vivo Evaluation of Clarithromycin/Poly(Lactic Acid) Microspheres for Intramuscular Drug Delivery. J. Contr. Rel. 1993, 26, 229–233. [Google Scholar]
- Oppenheim, R. C.; Stewert, N. F.; Gordon, L.; Patel, H. M. The Production and Evaluation of Orally Administred Insulin Nanoparticles. Drug Dev. Ind. Pharm. 1982, 8, 531–546. [Google Scholar]
- Hillery, A. M.; Toth, I.; Florence, A. T. Co-Polymerised Peptide Particles (CPP) II: Oral Uptake of a Novel Co-Polymeric Nanoparticulate Delivery System for Peptides. J. Control. Rel. 1996, 42, 65–73. [Google Scholar]
- Hillery, A. M.; Toth, I.; Shaw, A. J.; Florence, A. T. Co-Polymerised Peptide Particles (CPP) I: Synthesis, Charaterisation and in Vitro Studies on a Novel Oral Nanoparticulate Delivery System. J. Control. Rel. 1996, 41, 271–281. [Google Scholar]
- Ford, J.; Woolfe, J.; Florence, A. T. Nanospheres of Cyclosporin A: Poor Oral Absorption in Dogs. Int J Pharm 1999, 183, 3–6. [Google Scholar]
- Damgé, C.; Michel, C.; Aprahamian, M.; Couvreur, P. New Approach for Oral Administration of Insulin With Polyalkylcyanoacrylate Nanocapsules As Drug Carrier. Diabetes 1988, 37, 246–251. [Google Scholar]
- Michel, C.; Aprahamian, M.; Defontaine, L.; Couvreur, P.; Damgé, C. The Effect of Site of Administration in Gastrointestinal Tract on the Absorption of Insulin From Nanocapsules in Diabetic Rats. J. Pharm. Pharmacol. 1991, 43, 1–5. [Google Scholar]
- Al-Khouri Fallouh, N.; Roblot-Treupel, L.; Fessi, H.; Devissaguet, J. P.; Puisieux, F. Development of a New Process for the Manufacture of Polyisobutylcyanoacrylate Nanocapsules. Int. J. Pharm 1986, 28, 125–132. [Google Scholar]
- Ammoury, N.; Fessi, H.; Devissaguet, J. P.; Dubrasquet, M.; Benita, S. Jejunal Absorption, Pharmacological Activity, and Pharmacokinetic Evaluation of Indomethacin-Loaded Poly(DL-Lactide) and Poly(Isobutyl-Cyanoacrylate) Nanocapsules in Rats. Pharm. Res. 1991, 8, 101–108. [Google Scholar]
- Fessi, H.; Devissaguet, J. P.; Puisieux, F.; Thies, C. Procédé De Préparation De Systèmes Colloïdaux Dispersibles D'Une Substance, Sous Forme De Nanoparticules. Brevet français 1986, 2, 608–998. [Google Scholar]
- Quintanar-Guerrero, D.; Allémann, E.; Doelker, E.; Fessi, H. Preparation and Characterization of Nanocapsules From Preformed Polymers by a New Process Based on Emulsification-Diffusion Technique. Pharm.Res. 1998, 15, 1056–1062. [Google Scholar]
- Vranckx, H.; Demoustier, M.; Deleers, M. A New Nanocapsule Formulation With Hydrophilic Core: Application to the Oral Administration of Salmon Calcitonin in Rats. Eur. J. Pharm. Biopharm. 1996, 42, 345–347. [Google Scholar]
- Lambert, G.; Fattal, E.; Pinto-Alphandary, H.; Gulik, A.; Couvreur, P. Polyisobutylcyanoacrylate Nanocapsules Containing an Aqueous Core As a Novel Colloidal Carrier for the Delivery of Oligonucleotides. Pharm.Res. 2000, 17, 707–714. [Google Scholar]
- Magenheim, B.; Benita, S. Nanoparticle Characterization: a Comprehensive Physicochemical Approach. S.T.P.Pharma 1991, 1, 221–241. [Google Scholar]
- de Chasteigner, S.; Fessi, H.; Cavé, G.; Devissaguet, J. P.; Puisieux, F. Gastro-Intestinal Tolerance Study of a Freeze-Dried Oral Dosage Form of Indomethacin-Loaded Nanocapsules. S. T. P. Pharma Sciences 1995, 5, 242–246. [Google Scholar]
- De Jaeghere, F.; Allémann, E.; Leroux, J. C.; Stevels, W.; Feijen, J.; Doelker, E.; Gurny, R. Formulation and Lyoprotection of Poly(Lactic Acid-Co-Ethylene Oxide) Nanoparticles: Influence on Physical Stability and in Vitro Cell Uptake. Pharm. Res. 1999, 16, 859–866. [Google Scholar]
- Kreuter, J. Peroral Administration of Nanoparticles. Adv. Drug Del. Rev. 1991, 7, 71–86. [Google Scholar]
- Volkheimer, G. Persorption of Particles: Physiology and Pharmacology. Adv. Pharmaco. Chemother. 1977, 14, 163–187. [Google Scholar]
- Cornes, J. S. Number, Size, and Distribution of Peyer's Patches in the Human Small Intestine. Gut 1965, 6, 225–233. [Google Scholar]
- Volkheimer, G.; Schulz, F. H.; Aurich, I.; Strauch, S.; Beuthin, K.; Wendlandt, H. Persorption of Particles. Digestion 1968, 1, 78–82. [Google Scholar]
- Volkheimer, G.; Schultz, F. H. The Phenomenon of Persorption. Digestion 1968, 1, 213–218. [Google Scholar]
- Volkheimer, G. Hematogenous Dissemination of Ingested Polyvinyl Chloride Particles. Proceed. Ann. N. Y. Acad. Sci. 1975, 246, 164–171. [Google Scholar]
- Volkheimer, G.; Schulz, F. H.; Hofmann, I.; Pieser, J.; Rack, O.; Rotenbaecher, W.; Schurig, B.; Teicher, G.; Weiss, B. The Effect of Drugs on the Rate of Persorption. Pharmacology 1968, 1, 8–14. [Google Scholar]
- Simecka, J. W. Mucosal Immunity of the Gastrointestinal Tract and Oral Tolerance. Adv. Drug Del. Rev. 1998, 34, 235–259. [Google Scholar]
- McGhee, J. R.; Mestecky, J.; Dertzbaugh, M. T.; Eldridge, J. H.; Hirasawa, M.; Kiyono, H. The Mucosal Immune System: From Fundamental Concepts to Vaccine Development. Vaccine 1997, 10, 75–81. [Google Scholar]
- Eldridge, J. H.; Gilley, R. M.; Staas, J. K.; Moldoveanu, Z.; Tice, T. R. Biodegradable Microspheres: Vaccine Delivery System for Oral Immunization. Curr. Top. Microbiol. Immunol. 1989, 146, 59–66. [Google Scholar]
- O'Hagan, D. T. Microparticles and Polymers for the Mucosal Delivery of Vaccines. Adv. Drug Del. Rev. 1998, 34, 305–320. [Google Scholar]
- Jepson, M. A.; Simmons, N. L.; O'Hagan, D. T.; Hirst, B. H. Comparison of Poly(DL-Lactide-Co-Glycolide) and Polystyrene Microsphere Targeting to Intestinal M Cells. J. Drug Targ. 1993, 1, 245–249. [Google Scholar]
- McClean, S.; Prosser, E.; Meehan, E.; O'Malley, D.; Clarke, N.; Ramtoola, Z.; Brayden, D. Binding and Uptake of Biodegradable Poly-DL-Lactide Micro- and Nanoparticles in Intestinal Epithelia. Eur. J. Pharm. Sci. 1998, 6, 153–163. [Google Scholar]
- Jeurissen, S. H. M.; Kraal, G.; Sminia, T. The Role of Peyer's Patches in Intestinal Humoral Immune Responses Is Limited to Memory Formation. Adv. Exp. Med. Biol. 1987, 216A, 257–265. [Google Scholar]
- Wells, C. L.; Maddaus, M. A.; Erlandsen, S. L.; Simmons, R. L. Evidence for the Phagocytic Transfer of Intestinal Particles in Dogs and Rats. Infect. Immun. 1988, 56, 278–282. [Google Scholar]
- Jani, P. U.; Haklbert, G. W.; Langridge, J.; Florence, A. T. Nanoparticles Uptake by the Rat Gastrointestinal Mucosa: Quantitation and Particle Size Dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar]
- LeFevre, M. E.; Hancock, D. C.; Joel, D. D. Intestinal Barrier to Large Particulates in Mice. J. Toxicol. Env. Health. 1980, 6, 691–704. [Google Scholar]
- Delie, F. Evaluation of Nano- and Microparticle Uptake by the Gastrointestinal Tract. Adv. Drug Del. Rev. 1998, 34, 221–233. [Google Scholar]
- Delie, F.; Rubas, W. Caco-2 Monolayers As a Tool to Examine Intestinal Uptake of Particulates. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 1996, 23, 149–150. [Google Scholar]
- Desai, M. P.; Labhasetva, V.; Walter, E.; Levy, R. L.; Amidon, G. L. The Mechanism of Uptake of Biodegradable Microparticles in Caco-2 Cells Is Size Dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar]
- Le Visage, C.; Couvreur, P.; Mysiakine, E.; Breton, P.; Bru, N.; Fattal, E. In Vitro and in Vivo Evaluation of Poly(Methylidene Malonate 2.1.2) Microparticles Behavior for Oral Administration. J. Drug Target. 2001, 9, 141–153. [Google Scholar]
- Couvreur, P.; Lenaerts, V.; Kante, B.; Roland, M.; Speiser, P. P. Oral and Parenteral Administration of Insulin Associated to Hydrolysable Nanoparticles. Acta Pharm. Technol. 1980, 26, 220–222. [Google Scholar]
- Damgé, C.; Michel, C.; Aprahamian, M.; Couvreur, P.; Devissaguet, J. P. Nanocapsules As Carriers for Oral Peptide Delivery. J. Contr. Rel. 1990, 13, 233–239. [Google Scholar]
- Aboubakar, M.; Couvreur, P.; Pinto-Alphandary, H.; Gouritin, B.; Lacour, B.; Farinotti, R.; Puisieux, F.; Vauthier, C. Insulin-Loaded Nanocapsules for Oral Administration: In Vitro and in Vivo Investigation. Drug Develop. Res. 2000, 49, 109–117. [Google Scholar]
- Damgé, C.; Vonderscher, J.; Marbach, P.; Pinget, M. Poly(Alkyl Cyanoacrylate) Nanocapsules As a Delivery System in the Rat for Octreotide, a Long-Acting Somatostatin Analogue. J. Pharm. Pharmacol. 1997, 49, 949–954. [Google Scholar]
- Watnasirichaikul, S.; Tucker, I. G.; Davies, N. M. Effect of Formulation Variables on Characteristics of Poly(Ethylcyanoacrylate) Nanocapsules Prepared From W/o Microemulsions. Int. J. Pharm. 2002, 235, 237–246. [Google Scholar]
- Carino, G. P.; Jacob, J. S.; Mathiowitz, E. Nanospheres Based Oral Insulin Delivery. J. Contr. Rel. 2000, 65, 261–269. [Google Scholar]
- Ma, X. Y.; Pan, G. M.; Lu, Z.; Hu, J. S.; Bei, J. Z.; Jia, J. H.; Wang, S. G. Preliminary Study of Oral Polylactide Microcapsulated Insulin in Vitro and in Vivo. Diabetes Obes. Metab. 2000, 2, 243–250. [Google Scholar]
- Eldridge, J. H.; Hammond, C. J.; Meulbroek, J. A.; Staas, J. K.; Gilley, R. M.; Tice, T. R. Controlled Vaccine Release in the Gut-Associated Lymphoid Tissues. 1. Orally Administered Biodegradable Microspheres Target the Peyer's Patches. J. Contr. Rel. 1990, 11, 205–214. [Google Scholar]
- Leroux, J. C.; Cozens, R.; Roesel, J. L.; Galli, B.; Doelker, E.; Kubel, F.; Gurny, R. Pharmacokinetics of a Novel HIV-1 Protease Inhibitor Incorporated into Biodegradable or Enteric Nanoparticles Following Intravenous and Oral Administration to Mice. J. Pharm. Sci. 1995, 84, 1388–1391. [Google Scholar]
- Sordet, F.; Aumjaud, Y.; Fessi, H.; Derouin, F. Assessment of the Activity of Atovaquone-Loaded Nanocapsules in the Treatment of Acute and Chronic Murine Toxoplasmosis. Parasite 1998, 5, 223–229. [Google Scholar]
- Kim, Y. I.; Fluckiger, L.; Hoffman, M.; Lartaud-Idjouadiene, I.; Atkinson, J.; Maincent, P. The Antihypertensive Effect of Orally Administered Nifedipine-Loaded Nanoparticles in Spontaneously Hypertensive Rats. Brit. J. Pharmacol. 1997, 120, 399–404. [Google Scholar]
- Barichello, J. M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of Hydrophilic and Lipophilic Drugs in PLGA Nanoparticles by the Nanoprecipitation Method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar]
- Hubert, B.; Atkinson, J.; Guerret, M.; Hoffman, M.; Devissaguet, J. P.; Maincent, P. The Preparation and Acute Antihypertensive Effects of a Nanocapsular Form of Darodipine, a Dihydropyridine Calcium Entry Blocker. Pharm Res 1991, 8, 734–8. [Google Scholar]
- Löbenberg, R.; Maas, J.; Kreuter, J. Improved Body Distribution of 14C-Labelled AZT Bound to Nanoparticles in Rats Determined by Radioluminography. J. Drug Targ. 1997, 5, 171–179. [Google Scholar]
- Beck, P.; Kreuter, J.; Muller, W. E. G.; Schatton, W. Improved Peroral Delivery of Avarol With Polybutylcyanoacrylate Nanoparticles. Eur. J. Pharm. Biopharm. 1994, 40, 134–137. [Google Scholar]
- Singh, U. V.; Udupa, N. Methotrexate Loaded Chitosan and Chitin Microspheres - in Vitro Characterization and Pharmacokinetics in Mice Bearing Ehrlich Ascite Carcinoma. J. Microencaps. 1998, 15, 581–594. [Google Scholar]
- Leroux, J. C.; Cozens, R. M.; Roesel, J. L.; Galli, B.; Doelker, E.; Gurny, R. pH-Sensitive Nanoparticles: an Effective Means to Improve the Oral Delivery of HIV-1 Protease Inhibitor in Dogs. Pharm. Res. 1996, 13, 485–487. [Google Scholar]
- Lowman, A. M.; Morishita, M.; Kajita, M.; Nagai, T.; Peppas, N. A. Oral Delivery of Insulin Using pH-Responsive Complexation Gels. J. Pharm. Sci. 1999, 88, 933–937. [Google Scholar]
- Nagareya, N.; Uchida, T.; Matsuyama, K. Preparation and Characterization of Enteric Microspheres Containing Bovine Insulin by a w/o/w Emulsion Solvent Evaporation Method. Chem. Pharm. Bull. 1998, 46, 1613–1617. [Google Scholar]
- Mathiowitz, E.; Jacob, J. S.; Jong, Y. S.; Carino, G. P.; Chickering, D. E.; Chatuvedi, P.; Santos, C. A.; Vijayaraghavan, K.; Montgomery, S.; Basset, M.; Morell, C. Biologically Erodable Microspheres As Potential Oral Drug Delivery Systems. Nature 1997, 386, 410–414. [Google Scholar] [CrossRef]
- Leone-Bay, A.; Santiago, N.; Achan, D.; Chaudhary, K.; DeMorin, F.; Falzarano, L.; Haas, S.; Kalbag, S.; Kaplan, D.; Leipold, H.; Lercara, C.; O'Toole, D.; Rivera, T.; Rosado, C.; Sarubbi, D.; Vuocolo, E.; Wang, N.; Milstein, S.; Baughman, R. A. N-Acylated α-Amino Acids As Novel Oral Delivery Agents for Proteins. J. Med. Chem. 1995, 38, 4263–4269. [Google Scholar]
- Leone-Bay, A.; McInnes, C.; Wang, N.; DeMorin, F.; Achan, D.; Lercara, C.; Sarubbi, D.; Haas, S.; Press, J.; Barantsevich, E.; O'Broin, B.; Milstein, S.; Paton, D. Microsphere Formation in a Series of Derivatized α-Amino Acids: Properties, Molecular Modeling, and Oral Delivery of Salmon Calcitonin. J. Med. Chem. 1995, 38, 4257–4262. [Google Scholar]
- Milstein, S. J.; Barantsevich, E. N.; Grechanovski, V. A.; Sarubbi, D. J. PH-Dependent Microspheres From Modified Soybean Protein Hydrolysate. J. Microencaps. 1996, 13, 651–665. [Google Scholar]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Delie, F.; Blanco-Príeto, M.J. Polymeric Particulates to Improve Oral Bioavailability of Peptide Drugs. Molecules 2005, 10, 65-80. https://doi.org/10.3390/10010065
Delie F, Blanco-Príeto MJ. Polymeric Particulates to Improve Oral Bioavailability of Peptide Drugs. Molecules. 2005; 10(1):65-80. https://doi.org/10.3390/10010065
Chicago/Turabian StyleDelie, Florence, and María José Blanco-Príeto. 2005. "Polymeric Particulates to Improve Oral Bioavailability of Peptide Drugs" Molecules 10, no. 1: 65-80. https://doi.org/10.3390/10010065





