Cerebrosides from the Roots of Serratula chinensis
Abstract
:Introduction
Results and Discussion
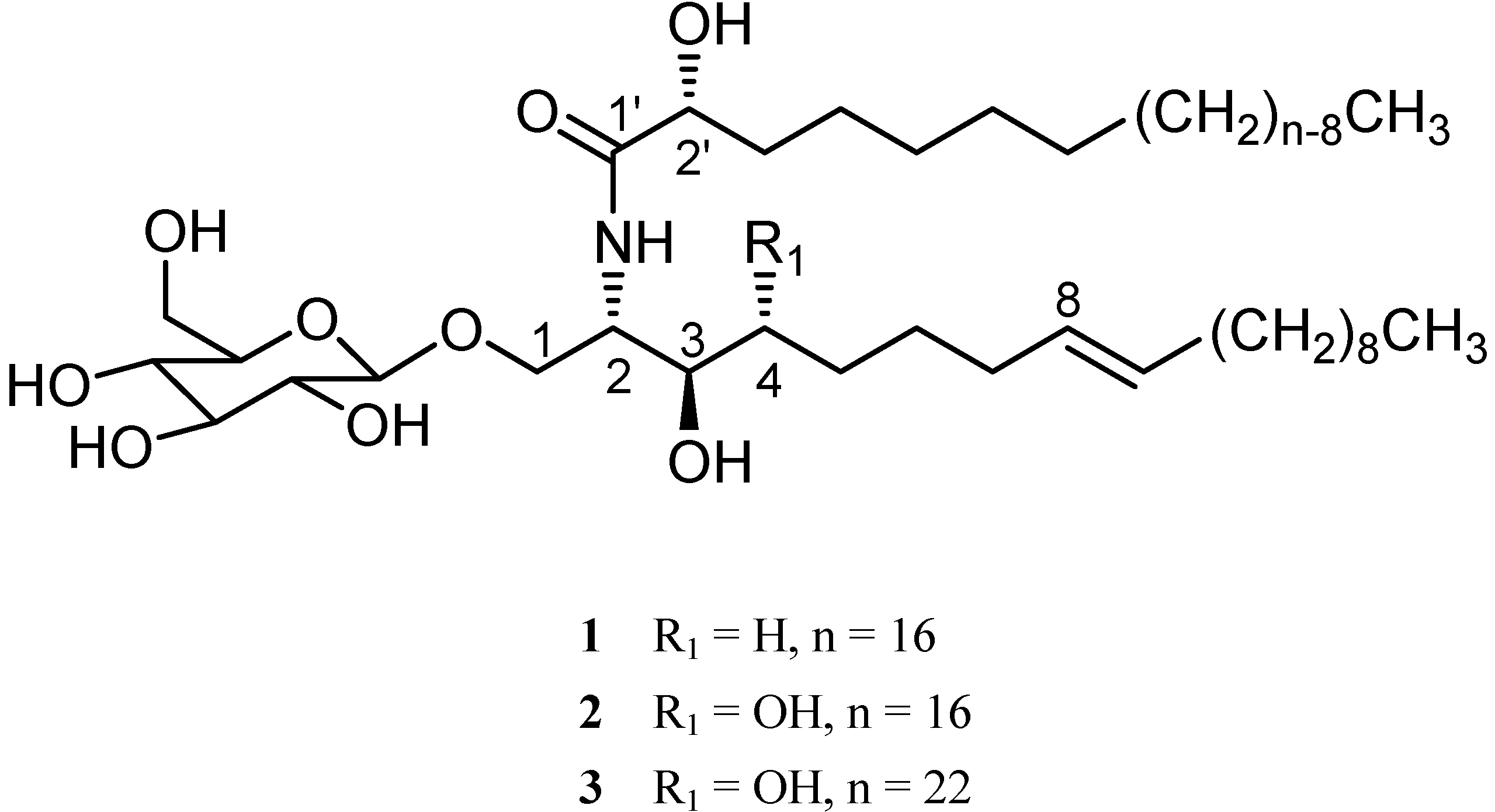
 + 8.8 (c 0.17, MeOH). Its positive ESI-MS showed a [M + K]+ peak at m/z 754, a [M + Na]+ peak at m/z 738 and a [M + H]+ peak at m/z 716, all in accordance with the molecular formula C40H77NO9. The IR spectrum showed strong absorption bands for hydroxyl (3430 cm-1), amide (1646 and 1540 cm-1) and (CH2)n (721 cm-1) groups. The 1H- and 13C-NMR spectra of 1 (Table 1) indicated the presence of a β-D-glucopyranosyl moiety (δH 4.86, 1H, d, J = 7.6 Hz, anomeric proton; δC 105.6, 75.1, 78.6, 71.7, 78.5, and 62.8), an amide linkage (δH 8.40, 1H, d, J = 9.2 Hz; δC 175.7), an amidomethine (δH 4.69, δC 54.6), an oxygenated methylene (δH 4.70 and 4.18; δC 70.4), two oxygenated methines (δH 4.16 and 4.59; δC 71.3 and 72.5), and two long chain aliphatic moieties. The above structural features indicated a dihydrosphingosine type cerebroside [5]. Methanolysis of 1 afforded a fatty acid methyl ester (FAME) and a long chain base (LCB) [6]. The FAME was identified as methyl 2-hydroxypalmitate by GC-MS analysis. The dihydrosphingosine moiety of 1 was derived as 2-amino-octadecene-1,3-diol by analysis of the 1H-1H COSY of 1 and the positive ESI-MS of the LCB. The absolute configuration of C-2′ was determined to be R form from the specific rotation of the FAME [7]. The 2S,3R stereochemistry was determined by comparison of the 13C-NMR chemical shifts of C-2 and C-3 with those of plakosides C and D [5,6]. In order to determine the position of the double bond in the dihydrosphingosine moiety, the KMnO4 oxidation was performed on the LCB [4]. The oxidation afforded n-decanoic acid which was determined by GC-MS analysis. This allowed the location of the double bond at C-8. The trans (E) configuration of the double bond in 1 was indicated by the olefinic proton signals which appeared as two double triplets (J = 14.4, 5.6 Hz) at δ 5.36 and 5.33 in CD3OD. This was supported by two carbon signals at δ 33.2/33.1 for the carbons next to the double bond in the 13C-NMR spectrum [8,9,10], which were assigned by the aid of 1H-1H COSY and HMQC. In conclusion, 1 was established to be 1-O-β-D-glucopyranosyl-(2S,3R,8E)-2-[(2’R)-2-hydroxylpalmitoylamino]-8-octadecene-1,3-diol.
+ 8.8 (c 0.17, MeOH). Its positive ESI-MS showed a [M + K]+ peak at m/z 754, a [M + Na]+ peak at m/z 738 and a [M + H]+ peak at m/z 716, all in accordance with the molecular formula C40H77NO9. The IR spectrum showed strong absorption bands for hydroxyl (3430 cm-1), amide (1646 and 1540 cm-1) and (CH2)n (721 cm-1) groups. The 1H- and 13C-NMR spectra of 1 (Table 1) indicated the presence of a β-D-glucopyranosyl moiety (δH 4.86, 1H, d, J = 7.6 Hz, anomeric proton; δC 105.6, 75.1, 78.6, 71.7, 78.5, and 62.8), an amide linkage (δH 8.40, 1H, d, J = 9.2 Hz; δC 175.7), an amidomethine (δH 4.69, δC 54.6), an oxygenated methylene (δH 4.70 and 4.18; δC 70.4), two oxygenated methines (δH 4.16 and 4.59; δC 71.3 and 72.5), and two long chain aliphatic moieties. The above structural features indicated a dihydrosphingosine type cerebroside [5]. Methanolysis of 1 afforded a fatty acid methyl ester (FAME) and a long chain base (LCB) [6]. The FAME was identified as methyl 2-hydroxypalmitate by GC-MS analysis. The dihydrosphingosine moiety of 1 was derived as 2-amino-octadecene-1,3-diol by analysis of the 1H-1H COSY of 1 and the positive ESI-MS of the LCB. The absolute configuration of C-2′ was determined to be R form from the specific rotation of the FAME [7]. The 2S,3R stereochemistry was determined by comparison of the 13C-NMR chemical shifts of C-2 and C-3 with those of plakosides C and D [5,6]. In order to determine the position of the double bond in the dihydrosphingosine moiety, the KMnO4 oxidation was performed on the LCB [4]. The oxidation afforded n-decanoic acid which was determined by GC-MS analysis. This allowed the location of the double bond at C-8. The trans (E) configuration of the double bond in 1 was indicated by the olefinic proton signals which appeared as two double triplets (J = 14.4, 5.6 Hz) at δ 5.36 and 5.33 in CD3OD. This was supported by two carbon signals at δ 33.2/33.1 for the carbons next to the double bond in the 13C-NMR spectrum [8,9,10], which were assigned by the aid of 1H-1H COSY and HMQC. In conclusion, 1 was established to be 1-O-β-D-glucopyranosyl-(2S,3R,8E)-2-[(2’R)-2-hydroxylpalmitoylamino]-8-octadecene-1,3-diol.Conclusions
Experimental
General
Plant material
Extraction and isolation
Cerebroside 1
 + 8.8 (c 0.17, MeOH). IR (KBr) cm−1: 3430, 2921, 2852, 1646, 1540, 1467, 1278, 1164, 1078, 721. 1H- and 13C-NMR data, see Table 1. Positive ESI-MS m/z: 754 [M + K]+, 738 [M + Na]+, 716 [M + H]+, 554 [M - Hexose + H]+.
+ 8.8 (c 0.17, MeOH). IR (KBr) cm−1: 3430, 2921, 2852, 1646, 1540, 1467, 1278, 1164, 1078, 721. 1H- and 13C-NMR data, see Table 1. Positive ESI-MS m/z: 754 [M + K]+, 738 [M + Na]+, 716 [M + H]+, 554 [M - Hexose + H]+.Methanolysis of 1
 -4.2 (c 0.01, CHCl3), GC-MS: GC tR 13.13 min, EI-MS m/z: 286 [M]+ (4), 268 [M - H2O]+ (0.2), 227 [M - CH3OCO]+ (9), 182 [M - CH3OCO - CH2OHCH2]+ (0.4), 159 (3), 145 (2), 127 [C9H19]+ (5), 125 (3), 111 (7), 97 (20), 90 [CH3OC(OH)=CHOH]+ (19), 83 (26), 69 (30), 57 (85). The H2O layer, after evaporation of MeOH, was adjusted to pH 9 with aqueous ammonia and extracted with Et2O. The Et2O layer was dried over anhydrous Na2SO4 and evaporated to yield the LCB 2-aminooctadec-8-ene-1,3-diol (0.7 mg); positive ESI-MS m/z: 300 [M + H]+, 282 [M - H2O + H]+.
-4.2 (c 0.01, CHCl3), GC-MS: GC tR 13.13 min, EI-MS m/z: 286 [M]+ (4), 268 [M - H2O]+ (0.2), 227 [M - CH3OCO]+ (9), 182 [M - CH3OCO - CH2OHCH2]+ (0.4), 159 (3), 145 (2), 127 [C9H19]+ (5), 125 (3), 111 (7), 97 (20), 90 [CH3OC(OH)=CHOH]+ (19), 83 (26), 69 (30), 57 (85). The H2O layer, after evaporation of MeOH, was adjusted to pH 9 with aqueous ammonia and extracted with Et2O. The Et2O layer was dried over anhydrous Na2SO4 and evaporated to yield the LCB 2-aminooctadec-8-ene-1,3-diol (0.7 mg); positive ESI-MS m/z: 300 [M + H]+, 282 [M - H2O + H]+.Oxidation of the LCB from 1
| Position | 1 | 3 | |||||
|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | ||||
| 1a | 4.70 m | 70.4 | 4.71 dd (10.4, 6.8) | 70.4 | |||
| 1b | 4.18 m | 4.51 dd (10.4, 4.0) | |||||
| 2 | 4.69 m | 54.6 | 5.30 m | 51.7 | |||
| 3 | 4.16 m | 71.3 | 4.29 m | 76.0 | |||
| 4 | 2.10 m | 34.9 | 4.20 m | 72.5 | |||
| 5 | 1.78 m | 25.9 | 2.10 m | 34.0 | |||
| 6 | 1.27-1.60 | 29.7-30.5 | 1.70 m | 25.9 | |||
| 7 and 10 | 2.10 m | 33.2 33.1 | 2.10 m | 33.4 33.1 | |||
| 8 and 9 | 5.47 m a) | 130.8 | 5.48 m b) | 130.9 130.7 | |||
| 11-15 | 1.27-1.60 | 29.7-30.5 | 1.23-1.32 | 29.7-30.1 | |||
| 16 | 1.27-1.60 | 32.3 | 1.23-1.32 | 32.2 | |||
| 17 | 1.27-1.60 | 23.1 | 1.23-1.32 | 23.0 | |||
| 18 | 0.88 t (6.8) | 14.4 | 0.86 t (6.8) | 14.4 | |||
| NH | 8.40 d (9.2) | 8.58 d (9.2) | |||||
| 1′ | 175.7 | 175.8 | |||||
| 2′ | 4.59 dd (6.8, 3.6) | 72.5 | 4.58 dd (7.6, 3.2) | 72.5 | |||
| 3′ | 2.10 m | 35.7 | 2.10 m | 35.6 | |||
| 4′ | 2.10 m, 1.78 m | 26.3 | 2.10 m, 1.70 m | 26.9 | |||
| 5′-(n - 3)′ | 1.27-1.60 | 29.7-30.5 | 1.23-1.32 | 29.7-30.1 | |||
| (n - 2)′ | 1.27-1.60 | 32.3 | 1.23-1.32 | 32.2 | |||
| (n - 1)′ | 1.27-1.60 | 23.1 | 1.23-1.32 | 23.0 | |||
| n′ | 0.88 t (6.8) | 14.4 | 0.86 t (6.8) | 14.4 | |||
| 1″ | 4.86 d (7.6) | 105.6 | 4.94 d (7.6) | 105.5 | |||
| 2″ | 3.98 t (8.0) | 75.1 | 4.00 t (8.0) | 75.2 | |||
| 3″ | 4.18 m | 78.6 | 4.20 m | 78.6 | |||
| 4″ | 4.18 m | 71.7 | 4.20 m | 71.6 | |||
| 5″ | 3.87 m | 78.5 | 3.87 m | 78.5 | |||
| 6″a | 4.48 br d (11.6) | 62.8 | 4.48 dd (11.6, 2.0) | 62.7 | |||
| 6″b | 4.30 dd (11.6, 5.6) | 4.30 dd (11.6, 6.8) | |||||
| a): δ 5.36 and 5.33 (each dt, J = 14.4, 5.6 Hz) when measured in CD3OD. b): δ 5.35 and 5.32 (each dt, J = 14.8, 4.8 Hz) when measured in CD3OD. n = 16 for 1; n = 22 for 3. | |||||||
Cerebroside 3
 + 9.4 (c 0.11, MeOH); IR (KBr) cm−1: 3400, 2923, 2854, 1722, 1643, 1537, 1466, 1381, 1261, 1167, 1078, 720; 1H- and 13C-NMR data, see Table 1; positive ESI-MS m/z: 854 [M + K]+, 838 [M + Na]+, 816 [M + H]+, 654 [M - hexose + H]+ .
+ 9.4 (c 0.11, MeOH); IR (KBr) cm−1: 3400, 2923, 2854, 1722, 1643, 1537, 1466, 1381, 1261, 1167, 1078, 720; 1H- and 13C-NMR data, see Table 1; positive ESI-MS m/z: 854 [M + K]+, 838 [M + Na]+, 816 [M + H]+, 654 [M - hexose + H]+ .Methanolysis of 3
 - 3.7 (c 0.01, CHCl3); GC-MS: GC tR 27.03 min; EI-MS m/z 370 [M]+ (6), 352 [M - H2O]+ (0.5), 311 [M - CH3OCO]+ (7), 266 [M - CH3OCO - CH2OHCH2]+ (1), 159 (2), 145 (3), 127 [C9H19]+ (8), 125 (5), 111 (13), 97 (28), 90 [CH3OC(OH)=CHOH]+ (25), 83 (30), 69 (31), 57 (88). The LCB, 2-amino-octadec-8-ene-1,3-diol, had the following ESI-MS: m/z 316 [M + H]+, 298 [M - H2O + H]+, 280 [M - 2H2O + H]+, 262 [M - 3H2O + H]+.
- 3.7 (c 0.01, CHCl3); GC-MS: GC tR 27.03 min; EI-MS m/z 370 [M]+ (6), 352 [M - H2O]+ (0.5), 311 [M - CH3OCO]+ (7), 266 [M - CH3OCO - CH2OHCH2]+ (1), 159 (2), 145 (3), 127 [C9H19]+ (8), 125 (5), 111 (13), 97 (28), 90 [CH3OC(OH)=CHOH]+ (25), 83 (30), 69 (31), 57 (88). The LCB, 2-amino-octadec-8-ene-1,3-diol, had the following ESI-MS: m/z 316 [M + H]+, 298 [M - H2O + H]+, 280 [M - 2H2O + H]+, 262 [M - 3H2O + H]+.Oxidation of the LCB from 3
Acknowledgments
References
- Shi, Z. Flora Reipulicae Popularis Sinicae; Science Press: Beijing, 1987; Tomus 78; (1), pp. 166–167. [Google Scholar]
- Jiangsu New Medical College. Dictionary of Chinese Crude Drugs; Shanghai Science and Technology Press: Shanghai, 1977; pp. 234–235. [Google Scholar]
- Ling, T. J.; Ma, W. Z.; Wei, X. Y. Ecdysteroids from the roots of Serratula chinensis. J. Trop. Subtrop. Bot. 2003, 11, 143–147. [Google Scholar]
- Ling, T. J.; Wu, P.; Liu, M. F.; Wei, X. Y. Ceramides from the roots of Serratula chinensis. J. Trop. Subtrop. Bot. 2005, 13, 403–407. [Google Scholar]
- Costantino, V.; Fattorusso, E.; Mangoni, A. Glycolipids from sponges. Part 9:1 plakoside C and D, two further prenylated glycosphingolipids from the marine sponge Ectyoplasia ferox. Tetrahedron 2000, 56, 5953–5957. [Google Scholar] [CrossRef]
- Kang, S. S.; Kim, J. S.; Son, K. H.; Kim, H. P.; Chang, H. W. Cyclooxygenase-2 inhibitory cerebrosides from Phytolaccae Radix. Chem. Pharm. Bull. 2001, 49, 321–323. [Google Scholar] [CrossRef]
- Shibuya, H.; Kawashima, K.; Sakagami, M.; Shimomura, M.; Ohashi, K.; Kitagawa, I. Sphingolipids and glycerolipids. Ι. Chemical structures and ionophoretic activities of soyacerebrosides Ι and ΙΙ form soybean. Chem. Pharm. Bull. 1990, 38, 2933–2938. [Google Scholar] [CrossRef]
- Fusetani, N.; Yasumuro, K.; Matsunaga, S. Haliclamines A and B, cytotoxic macrocyclic alkaloids from a sponge of the genus haliclona. Tetrahedron Lett. 1989, 30, 6891–6894. [Google Scholar] [CrossRef]
- Jung, J. H.; Lee, C. O.; King, Y. C.; Kang, S. S. New bioactive cerebrosides from Arisaema amurense. J. Nat. Prod. 1996, 59, 319–322. [Google Scholar] [CrossRef]
- Liu, H.; Orjala, J.; Rali, T.; Sticher, O. Glycosides from Stenochlaena palustris. Phytochemistry 1998, 49, 2403–2408. [Google Scholar] [CrossRef]
- Yamauchi, R.; Aizawa, K.; Inakuma, T.; Kato, K. Analysis of molecular species of glycolipids in fruit pastes of red bell pepper (Capsicum annuum L.) by high-performance liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2001, 49, 622–627. [Google Scholar] [CrossRef]
- Kang, S. S.; Kim, J. S.; Xu, Y. N.; Kim, Y. H. Isolation of a new cerebroside from the root bark of Aralia elata. J. Nat. Prod. 1999, 62, 1059–1060. [Google Scholar] [CrossRef]
- Tan, R. X.; Chen, J. H. The cerebrosides. Nat. Prod. Rep. 2003, 20, 509–534. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compound 1 are available from the authors.
© 2006 by MDPI (http:www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Ling, T.; Xia, T.; Wan, X.; Li, D.; Wei, X. Cerebrosides from the Roots of Serratula chinensis. Molecules 2006, 11, 677-683. https://doi.org/10.3390/11090677
Ling T, Xia T, Wan X, Li D, Wei X. Cerebrosides from the Roots of Serratula chinensis. Molecules. 2006; 11(9):677-683. https://doi.org/10.3390/11090677
Chicago/Turabian StyleLing, Tiejun, Tao Xia, Xiaochun Wan, Daxiang Li, and Xiaoyi Wei. 2006. "Cerebrosides from the Roots of Serratula chinensis" Molecules 11, no. 9: 677-683. https://doi.org/10.3390/11090677





