Synthesis and Bioevaluation of 5-Fluorouracil Derivatives
Abstract
:Introduction
Results and Discussion
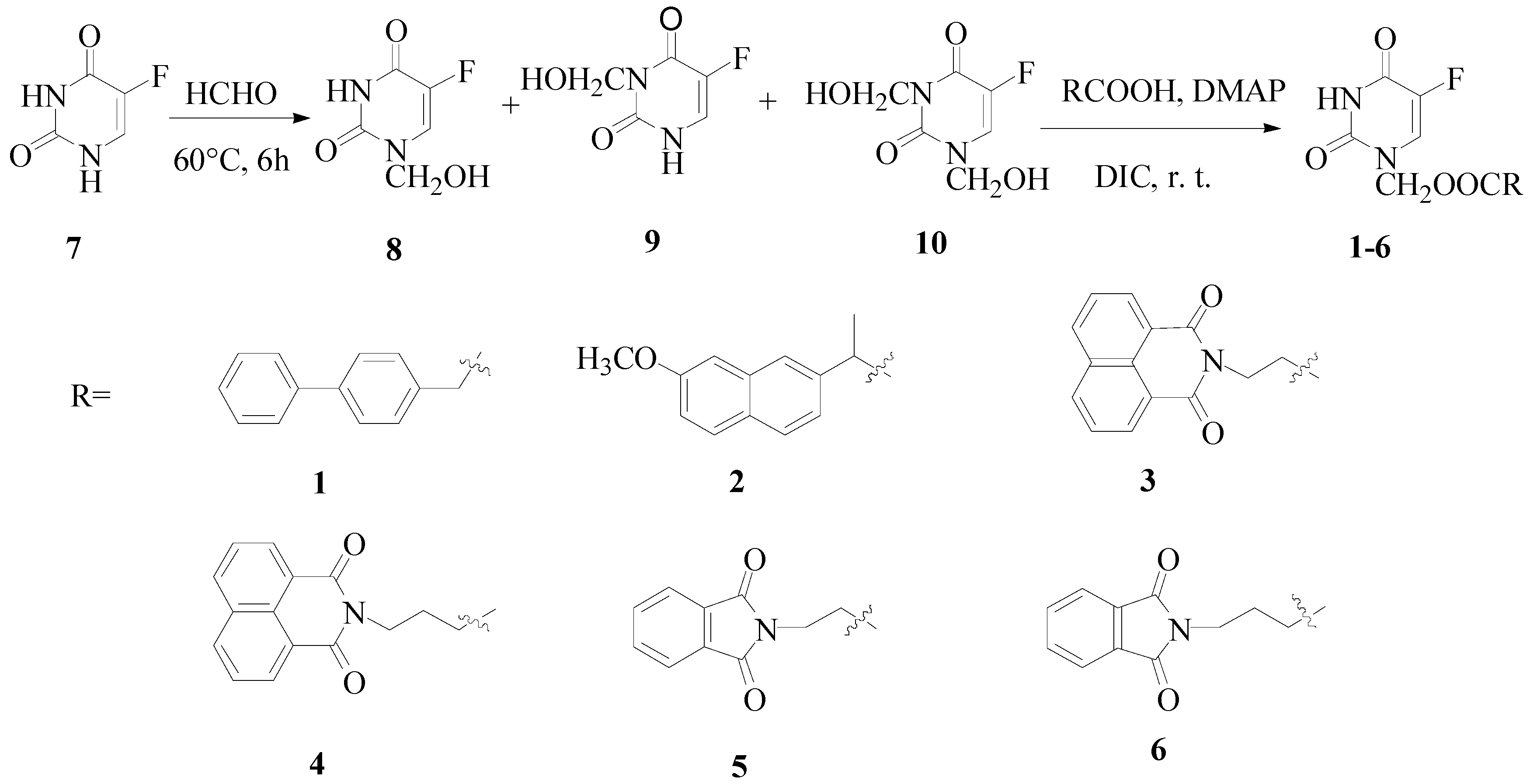
Chemistry
In vitro antitumor activity
| Sample | IC50 (µM) | ||
|---|---|---|---|
| K562 | B16 | CHO | |
| 5-Fu | 0.27 | 0.23 | 0.35 |
| 1 | 16.83 | 12.15 | 12.86 |
| 2 | 43.25 | 36.93 | >50 |
| 3 | >50 | 3.72 | 32.30 |
| 4 | 30.46 | 4.44 | 16.83 |
| 5 | >50 | 3.97 | 7.41 |
| 6 | 14.48 | 7.35 | 6.86 |
In vivo antitumor activity
| Sample (T/C, %) | Dose (µmol/kg) | Weight of tumor (g) | Ratio of tumor inhibition |
|---|---|---|---|
| Control | 0 | 1.63±0.31 | 0 |
| 5-Fu | 150 | 0.59±0.19 | 64.07 |
| 2 | 150 | 0.95±0.30 | 41.64 |
| 5 | 150 | 0.89±0.53 | 45.17 |
| 6 | 150 | 1.26±0.54 | 22.85 |
Conclusions
Experimental
General
Synthesis of the target compounds 1-6
In vitro antitumor activity experiments
In vivo antitumor activity experiments
Hydrolysis experiments
Acknowledgments
References
- Heidelberger, C.; Chaudhuri, N. K.; Danneberg, P.; Mooren, D.; Griesbach, L. Fluorinated pyrimidines, a new class of tumor-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; James, R. D. Integrating the oral fluoropyrimidines into the management of advanced colorectal cancer. Eur. J. Cancer 2001, 37, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Watanabe, Y.; Hoshiko, T.; Mizuno, H.; Ishikawa, K.; Mori, H. 5-Fluorouracil derivatives (IV): Synthesis of antitumor active acyloxyalkyl-5-fluouracils. Chem. Pharm. Bull. 1984, 32, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F. M.; Yao, X. J.; Tian, X.; Tu, Y. Q. Synthesis and Biological Evaluation of New 4β-5-Fu-substituted 4'-Demethylepipodophyllotoxin Derivatives. Molecules 2006, 11, 849–875. [Google Scholar]
- Zhang, C. X.; Zhang, Z. B.; Chen, H. M.; Tang, C. C.; Chen, R. Y. synthesis of 1,2- and 1,3-Cyclic Phospholipid Conjugates of N1-(2-Furanidyl)-N3-(2-hydroxyethyl)-5-fluorouracil. Heteroatom. Chem. 1998, 9, 295–298. [Google Scholar] [CrossRef]
- Jung, E. Y.; Chung, I. D.; Lee, N. J.; Park, J. S.; Ha, C. S.; Cho, W. J. Syntheses, Antitumor Activities, and Antiangiogenesis of a Monomer and Its Medium Molecular Weight Polymers: Maleimidoethanoyl-5-fluorouracil and Its Polymers. J. Polym. Sci., Part A: Polym. Chem. 2000, 38, 1247–1256. [Google Scholar]
- Lee, J. S.; Jung, Y. J.; Kim, Y. M. Synthesis and Evaluation of N-Acyl-2-(5-Fluorouracil-1-yl)-D,L- Glycine as a Colon-Specific Prodrug of 5-Fluorouracil. J. Pharm. Sci. 2001, 90, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Menger, F. M.; Rourk, M. J. Synthesis and Reactivity of 5-Fluorouracil/Cytarabine Mutual Prodrugs. J. Org. Chem. 1997, 62, 9083–9088. [Google Scholar]
- McElhinney, R. S.; McCormick, J. E.; Bibby, M. C.; Double, J. A.; Radacic, M.; Dumont, P. Nucleoside Analogs. 14. The synthesis and antitumor activity in mice of molecular combinations of 5-fluorouracil and N-(2-chloroethyl)-N-nitrosourea moieties separated by a three-carbon chain. J. Med. Chem. 1996, 39, 1403–1412. [Google Scholar]
- Mori, M.; Hatta, H.; Nishimoto, S. Stereoelectronic effect on one-electron reductive release of 5-fluorouracil from 5-fluoro-1-(2´-oxocycloalky)uracils as a new class of radiation-activated antitumor prodrugs. J. Org. Chem. 2000, 65, 4641–4647. [Google Scholar] [CrossRef] [PubMed]
- Bill-Cai, T.; Tang, X.; Nagorski, J.; Brauschweiger, P. G.; Wang, P. G. Synthesis and cytotoxicity of 5-fluorouracil/diazeniumdiolate conjugates. Bioorg. Med. Chem. 2003, 11, 4971–4975. [Google Scholar]
- Domínguez, J. F.; Marchal, J. A.; Correa, A.; Carrillo, E.; Boulaiz, H.; Aránega, A.; Gallo, M. A.; Espinosa, A. Synthesis and evaluation of new 5-fluorouracil antitumor cell differentiating derivatives. Bioorg. Med. Chem. 2003, 11, 315–323. [Google Scholar]
- Díaz-Gavilán, M.; Gómez-Vidal, J. A.; Entrena, A.; Gallo, M. A.; Espinosa, A.; Campos, J. M. Study of the factors that control the ratio of the products between 5-fluorouracil, uracil, and tetrahydrobenzoxazepine O,O-acetals bearing electron-withdrawing groups on the nitrogen atom. J. Org. Chem. 2006, 71, 1043–1054. [Google Scholar]
- Liu, Z. F.; Stephen, R. Synthesis and release of 5-fluorouracil from poly (N-vinylpyrrolidinone) bearing 5-fluorouracil derivatives. J. Control. Rel. 2002, 81, 91–99. [Google Scholar]
- Shoichiro, O. Synthesis and antitumor activity of 5-fluorouracil derivatives. Medicinal Research Reviews 1996, 16, 51–86. [Google Scholar] [CrossRef] [PubMed]
- Malet-Martino, M.; Jolimaître, P.; Martino, R. The prodrugs of 5-fluorouracil. Curr. Med. Chem.- Anti-Cancer Agents. 2002, 2, 267–310. [Google Scholar]
- Campos, J.; Dominguez, J. F.; Gallo, M. A.; Espinosa, A.; Campos, J.; Dominguez, J. F.; Gallo, M. A.; Espinosa, A. From a Classic Approach in Cancer Chemotherapy Towards Differentiation Therapy: Acyclic and Cyclic Seven-Membered 5-Fluorouracil O, N-Acetals. Curr. Pharmaceut. Design. 2000, 6, 1797–1810. [Google Scholar]
- Marx, J. L. Drug resistance of cancer cells probed. Science 1986, 234, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. X.; Zhao, J.; Sun, X. Q.; Wang, C. J. Synthesis, Interaction with DNA and Bioactivity of N-Piperazinoalkylamide. Chin. J. Org. Chem. 2006, 26, 1066–1072. [Google Scholar]
- Braña, M. F.; Castellano, J. M.; Perron, D.; Maher, C.; Conlon, D.; Bousquet, P. F.; George, J.; Qian, X.-D.; Robinson, S. P. Chromophore-Modified Bis-Naphthalimides: Synthesis and Anti-tumor Activity of Bis-Dibenz[de,h]isoquinoline-1,3-diones. J. Med. Chem. 1997, 40, 449–454. [Google Scholar]
- Lee, N. J.; Kim, K. H.; Rhew, H.Y.; Choi, W. M.; Chung, I. D.; Cho, W. J. Synthesis and biological activity of medium molecular weight polymers containing 3,6-endo-methylene-1,2,3,6-tetrahydrophthalimido-butanoyl-5-fluorouracil. Polym. Int. 2000, 49, 1702–1708. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tian, Z.-Y.; Du, G.-J.; Xie, S.-Q.; Zhao, J.; Gao, W.-Y.; Wang, C.-J. Synthesis and Bioevaluation of 5-Fluorouracil Derivatives. Molecules 2007, 12, 2450-2457. https://doi.org/10.3390/12112450
Tian Z-Y, Du G-J, Xie S-Q, Zhao J, Gao W-Y, Wang C-J. Synthesis and Bioevaluation of 5-Fluorouracil Derivatives. Molecules. 2007; 12(11):2450-2457. https://doi.org/10.3390/12112450
Chicago/Turabian StyleTian, Zhi-Yong, Gang-Jun Du, Song-Qiang Xie, Jin Zhao, Wen-Yuan Gao, and Chao-Jie Wang. 2007. "Synthesis and Bioevaluation of 5-Fluorouracil Derivatives" Molecules 12, no. 11: 2450-2457. https://doi.org/10.3390/12112450




