Bis(4-hydroxybenzyl)sulfide: a Sulfur Compound Inhibitor of Histone Deacetylase Isolated from Root Extract of Pleuropterus ciliinervis
Abstract
:Introduction
Results and Discussion
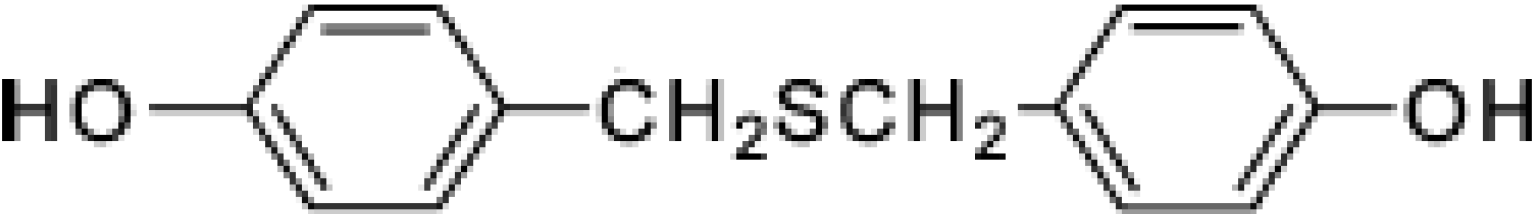
| Compound | IC50 (μM) Enzyme | GI50 (μM) PC-3 |
|---|---|---|
| 1 | 1.43 ± 0.03 | 7.65 ± 0.05 |
| SAHA | 0.13 ± 0.01 | 0.74 ± 0.01 |
| Cell line | Origin | Growth inhibition (μM) | |
|---|---|---|---|
| 1 | SAHA | ||
| ACHN | Kidney | 6.23 ± 0.07 | 0.67 ± 0.02 |
| NCI-H23 | Lung | >10 | 1.32 ± 0.02 |
| PC-3 | Prostate | 7.86 ± 0.06 | 0.75 ± 0.02 |
| MDA-MB-231 | Breast | 1.45 ± 0.03 | 0.89 ± 0.02 |
| LOX-IMVI | Melanoma | 6.14 ± 0.08 | 1.67 ± 0.02 |
| HCT-15 | Colon | 7.89 ± 0.12 | 0.93 ± 0.06 |
Conclusions
Experimental
General
Plant material collection, extraction and purification
Cell Culture
MTT Assay
Histone deacetylase assay
Statistical analysis
Acknowledgments
References
- Vigushin, D.M.; Coombes, R.C. Histone deacetylase inhibitors in cancer treatment. Anti-Cancer Drugs. 2002, 13, 1–13. [Google Scholar]
- Mai, A.; Massa, S.; Rotili, D.; Cerbara, L.; Valente, S.; Pezzi, R.; Simeoni, S.; Ragno, R. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med. Res. Rev. 2005, 25, 261–309. [Google Scholar]
- Imre, G.; Gekeler, V.; Leja, A.; Beckers, T.; Boehm, M. Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappaB by tumor necrosis factor-alpha receptor-1 down-regulation. Cancer Res. 2006, 66, 5409–5418. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.M.; Coombes, R.C. Targeted histone deacetylase inhibition for cancer therapy. Curr. Cancer Drug Targets 2004, 4, 205–18. [Google Scholar]
- Han, J.W.; Ahn, S.H.; Park, S.H.; Wang, S.Y.; Bae, G.U.; Seo, D.W.; Kwon, H.K.; Hong, S.; Lee, H.Y.; Lee, Y.W.; Lee, H.W. Apicidin, a histone deacetylase inhibitor, inhibits proliferation of tumor cells via induction of p21WAF1/Cip1 and gelsolin. Cancer Res. 2000, 60, 6068–6074. [Google Scholar]
- Kijima, M.; Yoshida, M.; Sugita, K.; Horinouchi, S.; Beppu, T. Trapoxin, an antitumor cyclic tetrapeptide, is an irreversible inhibitor of mammalian histone deacetylase. J. Biol. Chem. 1993, 268, 22429–22435. [Google Scholar]
- Ueda, H.; Manda, T.; Matsumoto, S.; Mukumoto, S.; Nishigaki, F.; Kawamura, I.; Shimomura, K. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. III. Antitumor activities on experimental tumors in mice. J. Antibiot. 1994, 47, 315–323. [Google Scholar]
- Butler, L.M.; Agus, D.B.; Scher, H.I.; Higgins, B.; Rose, A.; Cordon-Cardo, C.; Thaler, H.T.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 5165–5170. [Google Scholar]
- Mai, A.; Massa, S.; Ragno, R.; Cerbara, I.; Jesacher, F.; Loidl, P.; Brosch, G. 3-(4-Aroyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-alkylamides as a new class of synthetic histone deacetylase inhibitors. 1. Design, synthesis, biological evaluation, and binding mode studies performed through three different docking procedures. J. Med. Chem. 2003, 46, 512–524. [Google Scholar]
- Namba, T. The Encyclopedia of Wakan-Yaku (Traditional Sino-Japanese Medicines) with Color Pictures; Hoikusha Publishing Co, Ltd.: Osaka, Japan, 1993; p. 112. [Google Scholar]
- Teguo, P.W.; Fauconneau, B.; Deffieus, G.; Huguet, F.; Vercauteren, F.; Merillon, J.M. Isolation, identification, and antioxidant activity of three stilbene glucosides newly extracted from vitis vinifera cell cultures. J. Nat. Prod. 1998, 61, 655–657. [Google Scholar]
- Yoshizaki, M.; Fujino, H.; Arise, A.; Ohmura, K.; Arisawa, M.; Morita, N. Polygoacetophenoside, A New Acetophenone Glucoside from Polygonum multiflorum. Planta Med. 1987, 53, 273–275. [Google Scholar]
- Merillon, J.M.; Fauconneau, B.; Teguo, P.W.; Barrier, L.; Vercauteren, J.; Huguet, F. Antioxidant activity of the stilbene astringin, newly extracted from Vitis vinifera cell cultures. Clin Chem. 1997, 43, 1092–1093. [Google Scholar] [PubMed]
- Likhitwitayawuid, K.; Sritularak, B. A new dimeric stilbene with tyrosinase inhibitiory activity from Artocarpus gomezianus. J. Nat. Prod. 2001, 64, 1457–1459. [Google Scholar]
- Xiao, Y.Q.; Li, L.; You, X.L. Studies on chemical constituents of effective part of Gastrodia elata. China J. Chin. Materia Med. 2002, 27, 35–36. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Son, I.H.; Lee, S.I.; Yang, H.D.; Moon, H.-I. Bis(4-hydroxybenzyl)sulfide: a Sulfur Compound Inhibitor of Histone Deacetylase Isolated from Root Extract of Pleuropterus ciliinervis. Molecules 2007, 12, 815-820. https://doi.org/10.3390/12040815
Son IH, Lee SI, Yang HD, Moon H-I. Bis(4-hydroxybenzyl)sulfide: a Sulfur Compound Inhibitor of Histone Deacetylase Isolated from Root Extract of Pleuropterus ciliinervis. Molecules. 2007; 12(4):815-820. https://doi.org/10.3390/12040815
Chicago/Turabian StyleSon, Il Hong, Sung Ik Lee, Hyun Duk Yang, and Hyung-In Moon. 2007. "Bis(4-hydroxybenzyl)sulfide: a Sulfur Compound Inhibitor of Histone Deacetylase Isolated from Root Extract of Pleuropterus ciliinervis" Molecules 12, no. 4: 815-820. https://doi.org/10.3390/12040815




