Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum
Abstract
:Introduction

Results and Discussion
| Extract and fractions | IC50 (μg/ml ± SD)a | ||
|---|---|---|---|
| A549 | DLD-1 | WS1 | |
| Methanol | 15 ± 3 | 19.6 ± 0.9 | 10.6 ± 0.8 |
| Spirostanol | 8.8 ± 0.4 | 11.1 ± 0.6 | 6.3 ± 0.3 |
| Furostanol | 92 ± 19 | > 200 | 32 ± 2 |
| Aglycone | Sugar moities | ||||
|---|---|---|---|---|---|
| Position | δCb | δHc | Position | δCb | δHc |
| 1 | 46.9 t | 2.20 m | Galactose | ||
| 1.25 m | 1 | 102.7 d | 5.00 d (7.8) | ||
| 2 | 70.5 d | 4.11 m | 2 | 72.4 d | 4.53 m |
| 3 | 84.2 d | 4.06 m | 3 | 75.1 d | 4.18 m |
| 4 | 31.5 t | 2.39 q-like (12.6) | 4 | 79.5 d | 4.56 m |
| 2.16 m | 5 | 75.5 d | 4.11 m | ||
| 5 | 47.6 d | 1.15 m | 6 | 60.9 d | 4.54 m, 4.30 m |
| 6 | 69.9 d | 4.01 m | Glucose A | ||
| 7 | 40.4 t | 2.05 m | 1 | 103.9 d | 5.10 d (7.8) |
| 1.16 m | 2 | 80.4 d | 4.26 m | ||
| 8 | 31.9 d | 2.11 m | 3 | 88.0 d | 4.13 m |
| 9 | 54.3 d | 0.71 m | 4 | 70.2 d | 3.80 m |
| 10 | 36.9 s | - | 5 | 77.2 d | 3.82 m |
| 11 | 21.3 t | 1.52 m | 6 | 62.5 d | 4.43 m, 4.00 m |
| 1.37 m | Glucose B | ||||
| 12 | 40.0 t | 1.67 m | 1 | 103.5 d | 5.64 d (7.9) |
| 1.08 m | 2 | 74.4 d | 4.09 m | ||
| 13 | 40.8 s | - | 3 | 87.6 d | 4.24 m |
| 14 | 56.1 d | 1.13 m | 4 | 69.5 d | 3.92 m |
| 15 | 32.1 t | 1.46 m | 5 | 77.5 d | 3.89 m |
| 1.23 m | 6 | 62.1 d | 4.38 m, 4.29 m | ||
| 16 | 81.2 d | 4.58 m | Glucose C | ||
| 17 | 62.8 d | 1.85 t (7.3) | 1 | 104.0 d | 5.22 d (7.8) |
| 18 | 16.6 q | 0.82 s | 2 | 75.1 d | 4.02 m |
| 19 | 17.0 q | 1.24 s | 3 | 77.9 d | 4.21 m |
| 20 | 42.0 d | 1.94 quint. (6.7) | 4 | 71.3 d | 4.07 m |
| 21 | 15.0 q | 1.15 d (6.9) | 5 | 78.3 d | 4.01 m |
| 22 | 109.4 s | - | 6 | 62.1 d | 4.54 m, 4.18 m |
| 23 | 31.7 t | 1.66 m | Glucose D | ||
| 24 | 29.1 t | 1.57 m | 1 | 105.0 d | 5.17 d (7.8) |
| 25 | 30.5 d | 1.58 m | 2 | 75.3 d | 4.03 m |
| 26 | 66.9 t | 3.62 m | 3 | 77.6 d | 4.19 m |
| 3.51 m | 4 | 71.3 d | 4.07 m | ||
| 27 | 17.3 q | 0.69 d (5.3) | 5 | 78.1 d | 3.93 m |
| 6 | 62.2 d | 4.50 m, 4.17 m | |||
| Aglycone | Sugar moities | ||||
|---|---|---|---|---|---|
| Position | δCb | δHc | Position | δCb | δHc |
| 1 | 47.3 t | 1.90 m | Galactose | ||
| 0.92 m | 1 | 102.9 d | 4.39 d (7.8) | ||
| 2 | 71.4 d | 3.66 m | 2 | 72.9 d | 3.66 m |
| 3 | 85.3 d | 3.48 m | 3 | 75.6 d | 3.52 m |
| 4 | 31.6 t | 1.83 m | 4 | 80.4 d | 4.02 br d (3.2) |
| 1.76 m | 5 | 75.5 d | 3.52 m | ||
| 5 | 48.5 d | 1.20 m | 6 | 61.2 d | 3.89 m |
| 6 | 71.6 d | 3.80 m | 3.65 m | ||
| 7 | 40.8 t | 1.83 m | Glucose A | ||
| 1.18 m | 1 | 104.8 d | 4.56 d (7.8) | ||
| 8 | 30.8 d | 1.96 m | 2 | 84.8 d | 3.48 m |
| 9 | 55.6 d | 0.78 m | 3 | 78.2 d | 3.56 m |
| 10 | 37.9 s | - | 4 | 71.7 d | 3.22 m |
| 11 | 22.2 t | 1.56 m | 5 | 78.0 d | 3.29 m |
| 1.44 m | 6 | 63.2 d | 3.88 m | ||
| 12 | 41.1 t | 1.76 m | 3.58 m | ||
| 1.18 m | Glucose B | ||||
| 13 | 41.8 s | - | 1 | 106.1 d | 4.67 d (7.6) |
| 14 | 57.2 d | 1.17 m | 2 | 76.2 d | 3.27 m |
| 15 | 32.8 t | 2.00 m | 3 | 77.7 d | 3.37 m |
| 1.29 m | 4 | 70.9 d | 3.37 m | ||
| 16 | 82.2 d | 4.39 m | 5 | 78.7 d | 3.35 m |
| 17 | 63.9 d | 1.75 m | 6 | 62.2 d | 3.97 m |
| 18 | 17.0 q | 0.82 s | 3.80 m | ||
| 19 | 17.3 q | 1.06 s | |||
| 20 | 43.0 d | 1.90 m | |||
| 21 | 14.9 q | 0.96 d (7.0) | |||
| 22 | 110.6 s | - | |||
| 23 | 32.5 t | 1.70 m | |||
| 1.56 m | |||||
| 24 | 29.9 t | 1.62 m | |||
| 1.42 m | |||||
| 25 | 31.5 d | 1.59 m | |||
| 26 | 67.9 t | 3.44 m | |||
| 3.32 m | |||||
| 27 | 17.5 q | 0.79 d (6.4) | |||
| Compounds | IC50 (μM ± SD) | ||
|---|---|---|---|
| A549 | DLD-1 | WS1 | |
| Yayoisaponin C (1) | 3.7 ± 0.7 | 5.6 ± 0.2 | 3.0 ± 0.2 |
| Eruboside B (2) | 5.3 ± 0.5 | 8.2 ± 0.4 | 3.6 ± 0.2 |
| Aginoside (3) | 5.8 ± 0.9 | 7.9 ± 0.5 | 3.6 ± 0.2 |
| Compound 4 | 9 ± 1 | 13 ± 1 | 3.1 ± 0.1 |
| Leucospiroside A (5) | 5.0 ± 0.1 | 7.2 ± 0.1 | 4.55 ± 0.07 |
| Compound 6 | 22 ± 2 | 22 ± 2 | 14.5 ± 0.5 |
| Compound 7 | 7.8 ± 0.4 | 8.9 ± 0.2 | 7.7 ± 0.2 |
| Etoposide | 1.1 ± 0.1 | 4.8 ± 0.8 | n.d. |
| 5-fluorouracil | 48 ± 18 | 11 ± 2 | 20 ± 2 |
Experimental Section
General experimental
Plant material
Extraction and isolation
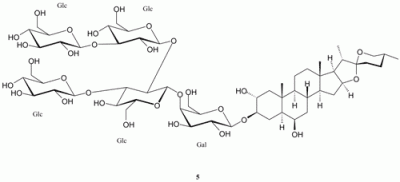
Spectroscopic data for leucospiroside A (5)
Acid hydrolysis of leucospiroside A (5)
Cell culture
Cytotoxicity assay
Acknowledgements
References and Notes
- Cholokashvili, N. Notulae Systematicae et Geographicae; Institute of Botany: Tbilisi, Republic of Georgia, 1977. [Google Scholar]
- Gagnidze, R. Vascular Plants of Georgia a Nomenclatural Checklist; Ketskhoveli, N., Ed.; Institute of Botany: Tbilisi, Republic of Georgia, 2005. [Google Scholar]
- Panaskerteli-Tsitsishvili, Z. Karabadini; Sabchota Sakartvelo: Tbilisi, Republic of Georgia, 1978. [Google Scholar]
- Bagrationi, D. Iadigar Daudi; Edition of Tbilisi University: Tbilisi, Republic of Georgia, 1993. [Google Scholar]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Kapadnis, B.P. Heat stable antimicrobial activity of Allium ascalonicum against bacteria and fungi. Indian J. Exp. Biol. 2005, 43, 751–754. [Google Scholar]
- Matsuura, H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 2001, 131, 1000S–1005S. [Google Scholar]
- Barile, E.; Bonanomi, G.; Antignani, V.; Zolfaghari, B.; Sajjadi, S.E.; Scala, F.; Lanzotti, V. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry 2007, 68, 596–603. [Google Scholar] [CrossRef]
- Zhang, Y. W.; Morita, I.; Shao, G.; Yoa, X. S.; Murota, S. Screening of anti-hypoxia/reoxygenation agents by an in vitro model. Part 1: Natural inhibitors for protein tyrosine kinase activated by hypoxia/reoxygenation in cultured human umbilical vein endothelial cells. Planta Med. 2000, 66, 114–118. [Google Scholar] [CrossRef]
- Stajner, D.; Milic-Demarino, N.; Canadanovic-Brunet, J.; Stajner, M.; Popovic, B. M. Screening for antioxidant properties of Allium giganteum. Fitoterapia 2006, 77, 268–270. [Google Scholar]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Di Rosa, M.; Ianaro, A. Cytotoxic saponins from bulbs of Allium porrum L. J. Agric. Food Chem. 2000, 48, 3455–3462. [Google Scholar] [CrossRef]
- Baba, M.; Ohmura, M.; Kishi, N.; Okada, Y.; Shibata, S.; Peng, J.; Yao, S.-S.; Nishino, H.; Okuyama, T. Saponins isolated from Allium chinense G. Don and antitumor-promoting activities of isoliquiritigenin and laxogenin from the same drug. Biol. Pharm. Bull 2000, 23, 660–662. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fattorusso, E.; Lanzotti, V.; Magno, S.; Carnuccio, R.; D’Acquisto, F. 12-Keto-porrigenin and the unique 2,3-seco-porrigenin, new antiproliferative sapogenins from Allium porrum. Tetrahedron 1997, 53, 3401–3406. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Ide, A.; Kameyama, A.; Yokosuka, A.; Sashida, Y. Steroidal saponins from the aerial parts of Dracaena draco and their cytostatic activity on HL-60 cells. Phytochemistry 1999, 50, 805–813. [Google Scholar] [CrossRef]
- Chincharadze, D. G.; Kelginbaev, A. N.; Gorovits, M. B.; Eristavi, L. I.; Gorovits, T. T.; Abubakirov, N. K. Steroidal saponins and sapogenins of Allium. 15. Eruboside-B from Allium erubescens. Khim. Prir. Soedin 1980, 509–514. [Google Scholar]
- ata, N.; Matsunaga, S.; Fusetani, N.; Nishikawa, H.; Takamura, S.; Saito, T. New antifungal and cytotoxic steroidal saponins from the bulbs of an elephant garlic mutant. Biosci., Biotechnol., Biochem 1998, 62, 1904–1911. [Google Scholar] [CrossRef]
- Kel’ginbaev, A. N.; Gorovits, M. B.; Gorovits, T. T.; Abubakirov, N. K. Steroidal saponins and sapogenins of Allium. 9. Aginoside structure. Khim. Prir. Soedin. 1976, 480–486. [Google Scholar]
- Mimaki, Y.; Kawashima, K.; Kanmoto, T.; Sashida, Y. Steroidal glycosides from Allium albopilosum and A. ostrowskianum. Phytochemistry 1993, 34, 799–805. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fattorusso, E.; Lanzotti, V.; Magno, S. Spirostanol saponins of Allium porrum L. Phytochemistry 1999, 51, 1077–1082. [Google Scholar] [CrossRef]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Fuwa, T. Further-studies on steroidal glycosides from bulbs, roots and leaves of Allium sativum L. Chem. Pharm. Bull. 1989, 37, 2741–2743. [Google Scholar] [CrossRef]
- Agrawal, P. K. NMR-spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Hayashi, N.; Fuwa, T. A furostanol glycosides from garlic, bulbs of Allium sativum L. Chem. Pharm. Bull. 1988, 36, 3659–3663. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1-7 are available from authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mskhiladze, L.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Kuchukhidze, J.; Elias, R.; Pichette, A. Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum. Molecules 2008, 13, 2925-2934. https://doi.org/10.3390/molecules13122925
Mskhiladze L, Legault J, Lavoie S, Mshvildadze V, Kuchukhidze J, Elias R, Pichette A. Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum. Molecules. 2008; 13(12):2925-2934. https://doi.org/10.3390/molecules13122925
Chicago/Turabian StyleMskhiladze, Lasha, Jean Legault, Serge Lavoie, Vakhtang Mshvildadze, Jumber Kuchukhidze, Riad Elias, and André Pichette. 2008. "Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum" Molecules 13, no. 12: 2925-2934. https://doi.org/10.3390/molecules13122925