Chemical Synthesis of Proanthocyanidins in Vitro and Their Reactions in Aging Wines
Abstract
:1. Introduction
2. Chemical synthesis of proanthocyanidins
2.1 Nonenzymatic synthesis of proanthocyanidin precursors

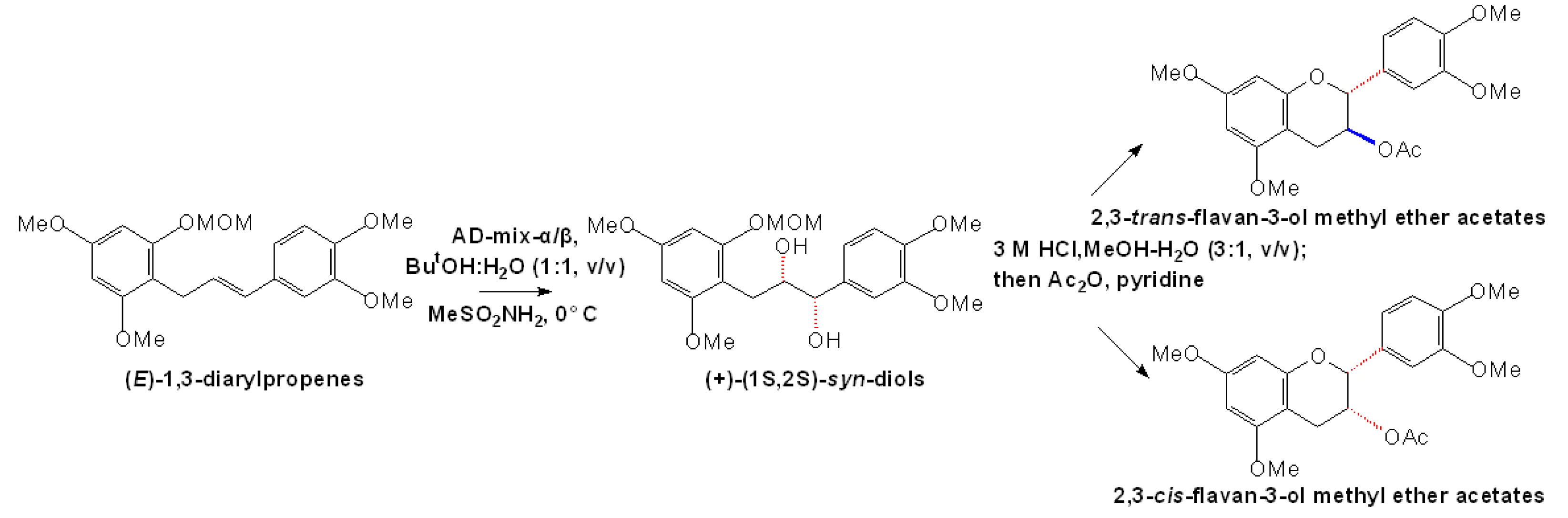
2.2 Chemical synthesis of proanthocyanidins with direct condensation of flavanols or their derivatives


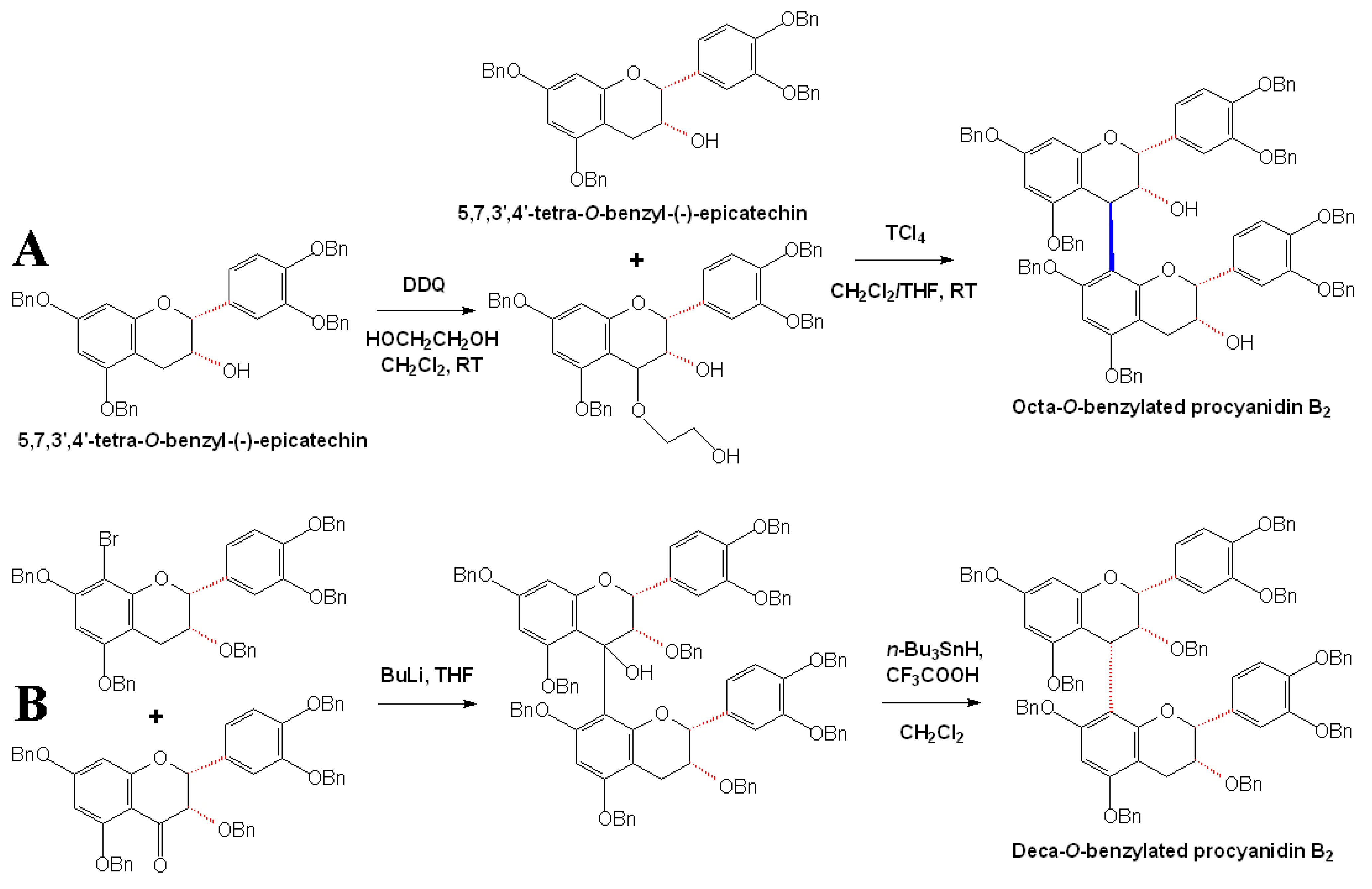
2.3 Stereoselective synthesis of B-type procyanidins




2.4 Synthesis of A-type Procyanidins

3. Reactions of Proanthocyanidins in Wine
3.1 Proanthocyanidins in wine
3.2 Direct reactions among flavanols, anthocyanins and proanthocyanidins

3.3 Indirect reactions among flavanols, anthocyanins and proanthocyanidins

3.4 Other Anthocyanins Derived Condensed Pigment
4. Conclusions and Perspectives
Acknowledgements
References and Notes
- Bruyne, T.D.; Pieters, L.; Deelstra, H.; Vlietinck, A. Condensed vegetable tannins: biodiversity in structure and biological activities. Biochem. Syst. Ecol. 1999, 27, 445–459. [Google Scholar] [CrossRef]
- Haggerman, A.E.; Buttler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar]
- Kennedy, J.A.; Powell, K.J. Polyphenol interactions with aluminium(III) and iron(III): their possible involvement in the podzolization process. Aust. J. Chem. 1985, 38, 879–888. [Google Scholar] [CrossRef]
- Weber, H.A.; Hodges, A.E.; Guthrie, J.R.; O’Brien, B.M.; Robaugh, D.; Clark, A.P.; Harris, R.K.; AlgaierL, J.W.; Smith, C.S. Comparison of proanthocyanidins in commercial antioxidants: grape seed and pine bark extracts. J. Agric. Food Chem. 2007, 55, 148–156. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Iijima, K.; Yoshizumi, M.; Ouchi, Y. Effect of red wine polyphenols on vascular smooth muscle cell function - molecular mechanism of the ‘French paradox’. Mech. Ageing Dev. 2002, 123, 1033–1039. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Marles, M.A.S.; Ray, H.; Gruber, M.Y. New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 2003, 64, 367–383. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins – a final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Ieri, F.; Goretti, M.; Branda, E.; Mulinacci, N.; Romani, A. Catechins and proanthocyanidins: naturally occurring O-heterocycles with antimicrobial activity. Top Heterocycl. Chem. 2007, 10, 239–263. [Google Scholar]
- Peyrot Des Gachons, C.; Kennedy, J.A. Direct method for determining seed and skin proanthocyanidin extraction into red wine. J. Agric. Food Chem. 2003, 51, 5877–5881. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueňas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Flucrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar]
- Sun, B.; Spranger, M.I. Changes in phenolic composition of Tinta Miúda red wines after 2 years of ageing in bottle: effect of winemaking technologies. Eur. Food Res. Technol. 2005, 221, 305–312. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo Da Silva, J.M. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar]
- Singleton, V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: Observations and practical implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar]
- Vernhet, A.; Pellerin, P.; Prieur, C.; Osmianski, J.; Moutounet, M. Charge properties of some grape and wine polysaccharide and polyphenolic fractions. Am. J. Enol. Vitic. 1996, 47, 25–29. [Google Scholar]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Martens, S.; Teeri, T.; Forkmann, G. Heterologous expression of dihydroflavonol 4-reductases from various plants. FEBS Lett. 2002, 531, 453–458. [Google Scholar] [CrossRef]
- Stafford, H.A.; Lester, H.H. Enzymic and nonenzymic reduction of (+)-dihydroquercetin to its 3,4,-diol. Plant Physiol. 1982, 70, 695–698. [Google Scholar]
- Kikuchi, T.; Nishimura, M.; Hoshino, A.; Morita, Y.; Iida, S.; Saito, N.; Honda, T. An efficient conversion of catechine into 3,4-trans-leucocyanidin. Heterocycles 2003, 60, 1469–1475. [Google Scholar] [CrossRef]
- Stafford, H.A.; Lester, H.H. Flavan-3-ol Biosynthesis. The conversion of (+)-dihydroquercetin and flavan-3,4-cis-diol (leucocyanidin) to (+)-catechin by reductases extracted from cell suspension cultures of Douglas fir. Plant Physiol. 1984, 76, 184–186. [Google Scholar] [CrossRef]
- Kristiansen, K.N. Conversion of (+)-dihydroquercetin to (+)-2,3-trans-3,4-cis-leucocyanidin and (+)-catechin with an enzyme extract from maturing grains of barley. Carlsberg Res. Comm. 1986, 51, 51–60. [Google Scholar] [CrossRef]
- Singh, S.; Mccallum, J.; Gruber, M.Y.; Towers, G.H.N.; Muir, A.D.; Bohm, B.A.; Koupai-Abyazani, M.R.; Glass, A.D.M. Biosynthesis of flavan-3-ols by leaf extracts of Onobrychis viciifolia. Phytochemistry 1997, 44, 425–432. [Google Scholar] [CrossRef]
- Van Rensburg, H.; Van Heerden, P.S.; Bezuidenhoudt, B.C.B.; Ferreira, D. Enantioselective synthesis of the four catechin diastereomer derivatives. Tetrahedron Lett. 1997, 38, 3089–3092. [Google Scholar]
- Creasey, L.L.; Swain, T. Structure of condensed tannins. Nature 1965, 208, 151–153. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Foo, L.Y. Condensed tannins: quinone methide intermediates in procyanidin synthesis. J. Chem. Soc., Chem. Commun. 1983, 1035–1036. [Google Scholar] [CrossRef]
- Foo, L.Y.; Hemingway, R.W. Condensed tannins: synthesis of the first branched procyanidin trimer. J. Chem. Soc., Chem. Commun. 1984, 85–86. [Google Scholar]
- Hemingway, R.W.; Laks, P.E. Condensed tannins: a proposed route to 2R,3R-(2,3-cis)-proanthocyanidins. J. Chem. Soc., Chem. Commun. 1985, 746–747. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 4. A direct biomimetic approach to [4,6]- and [4,8]-biflavanoids. J. Chem. Soc., Perkin Trans. 1 1981, 1235–1245. [Google Scholar] [CrossRef]
- Delcour, J.A.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 9. The condensation sequence of leucocyanidin with (+)-catechin and with the resultant procyanidins. J. Chem. Soc., Perkin Trans. 1 1983, 1711–1717. [Google Scholar] [CrossRef]
- Delcour, J.A.; Serneels, E.J.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 13. The first 2,3-trans-3,4-cis-procyanidins: sequence of units in a ‘trimer’ of mixed stereochemistry. J. Chem. Soc., Perkin Trans. 1 1985, 669–676. [Google Scholar]
- Steynberg, P.L.; Nel, R.J.J.; Van Rensburg, H,; Bezuidenhoudt, B.C.B.; Ferreira, D. Oligomeric flavanoids. Part 27. Interflavanyl bond formation in procyanidins under neutral conditions. Tetrahedron 1998, 54, 8153–8158. [Google Scholar] [CrossRef]
- Malan, J.C.S.; Steynberg, P.J.; Steynberg, J.P.; Young, D.A.; Bezuidenhoudt, B.B.C.; Ferreira, D. Oligomeric flavanoids. part 14. Proguibourtinidins based on (-)-fisetinidol and (+)-epifisetinidol units. Tetrahedron 1990, 46, 2883–2890. [Google Scholar] [CrossRef]
- Malan, J.C.S.; Steenkamp, J.A.; Young, D.A.; Ferreira, D. Oligomeric flavanoids. Part 13. Synthesis of profisetinidins based on (-)-robinetinidol and (+)-epifisetinidol. Tetrahedron 1989, 45, 7859–7868. [Google Scholar] [CrossRef]
- Viviers, P.M.; Botha, J.J.; Ferreira, D.; Roux, D.G.; Saayman, H.M. Synthesis of condensed tannins. Part 7. Angular [4,6 : 4,8]-prorobinetinidin triflavanoids from black wattle (‘Mimosa’) bark extract. J. Chem. Soc., Perkin Trans. 1 1983, 17–22. [Google Scholar]
- Bennie, L.; Malan, E.; Coetzee, J.; Ferreira, D. Structure and synthesis of ether-linked proteracacinidin and promelacacinidin proanthocyanidins from Acacia caffra. Phytochemistry 2000, 53, 785–793. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G.; Hull, W.E. Condensed tannins: condensation mode and sequence during formation of synthetic and natural triflavonoids. J. Chem. Soc., Chem. Commun. 1979, 510–512. [Google Scholar]
- Ferreira, D.; Bekker, R. Oligomeric proanthocyanidins: naturally occurring O-heterocycles. Nat. Prod. Rep. 1996, 13, 411–433. [Google Scholar] [CrossRef]
- Ferreira, D.; Li, X.C. Oligomeric proanthocyanidins: naturally occurring O-heterocycles. Nat. Prod. Rep. 2000, 17, 193–212. [Google Scholar] [CrossRef]
- Ferreira, D.; Slade, D. Oligomeric proanthocyanidins: naturally occurring O-heterocycles. Nat. Prod. Rep. 2002, 19, 517–541. [Google Scholar] [CrossRef]
- Kawamoto, H.; Nakatsubo, F.; Murakami, K. Synthesis of a condensed tannin model compound, 4-(2,4,6-trihydroxyphenyl)flavan-3,3',4',5,7-pentaol. J. Wood Chem. Technol. 1989, 9, 35–52. [Google Scholar] [CrossRef]
- Ballenegger, M.E.; Rimbault, C.G.; Albert, A.I.; Weith, A.J.; Courbat, P.; Tyson, R.G.; Palmer, D.R.; Thompson, D.G. Eur. Pat. Appl. EP 0096007, 1983.
- Saito, A.; Emoto, M.; Tanaka, A.; Doi, Y.; Shoji, K.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Matsuura, N.; Nakajima, N. Stereoselective synthesis of procyanidin B3-3-O-gallate and 3,3’’-di-O-gallate, and their abilities as antioxidant and DNA polymerase inhibitor. Tetrahedron 2004, 60, 12043–12049. [Google Scholar] [CrossRef]
- Arnaudinaud, V.; Nay, B.; Vergé, S.; Nuhrich, A.; Deffieux, G.; Mérillon, J.-M.; Monti, J.-P.; Vercauteren, J. Total synthesis of isotopically labelled flavonoids. Part 5: Gram-scale production of C-labelled (-)-procyanidin B3. Tetrahedron Lett. 2001, 42, 5669–5671. [Google Scholar] [CrossRef]
- Yoneda, S.; Kawamoto, H.; Nakatsubo, F. Synthesis of high molecular mass condensed tannin by cationic polymerization of flavan 3,4-carbonate. J. Chem. Soc., Perkin Trans. 1 1997, 1025–1030. [Google Scholar]
- Ohmori, K.; Ushimaru, N.; Suzuki, K. Oligomeric catechins: An enabling synthetic strategy by orthogonal activation and C(8) protection. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12002–12007. [Google Scholar] [CrossRef]
- Hundt, H.K.L.; Roux, D.G. Synthesis of condensed tannins. Part 3. Chemical shifts for determining the 6- and 8-bonding positions of ‘terminal of’ (+)-catechin units. J. Chem. Soc., Perkin Trans. 1 1981, 1227–1234. [Google Scholar]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic Studies of Proanthocyanidins. Highly stereoselective synthesis of the catechin dimer, procyanidin-B3. Biosci. Biotechnol. Biochem. 2002, 66, 1764–1767. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 2: Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron 2002, 58, 7829–7837. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 3: Stereoselective 3,4-cis catechin and catechin condensation by TMSOTf-catalyzed intramolecular coupling method. Tetrahedron Lett. 2003, 44, 5449–5452. [Google Scholar] [CrossRef]
- Saito, A.; Doi, Y.; Tanaka, A.; Matsuura, N.; Ubukata, M.; Nakajima, N. Systematic synthesis of four epicatechin series procyanidin trimers and their inhibitory activity on the Maillard reaction and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 4783–4790. [Google Scholar] [CrossRef]
- Tülckmantel, W.; Kozikowski, A.P.; Romanczyk Jr., L.J. Studies in polyphenol chemistry and bioactivity. 1. preparation of building blocks from (+)-catechin. procyanidin formation. Synthesis of the cancer cell growth inhibitor, 3-O-galloyl-(2R,3R)-epicatechin- 4β,8-[3-O-galloyl-(2R,3R)-epicatechin]. J. Am. Chem. Soc. 1999, 121, 12073–12081. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Tülckmantel, W.; Hu, Y. Studies in Polyphenol Chemistry and Bioactivity. 3. Stereocontrolled Synthesis of epicatechin-4α,8-epicatechin, an unnatural isomer of the B-type procyanidins. J. Org. Chem. 2001, 66, 1287–1296. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Tülckmantel, W.; Böttcher, G.; Romanczyk Jr., L.J. Studies in polyphenol chemistry and bioactivity. 4. Synthesis of trimeric, tetrameric, pentameric, and higher oligomeric epicatechin-derived procyanidins having all-4β,8-interflavan connectivity and their inhibition of cancer cell growth through cell cycle arrest. J. Org. Chem. 2003, 68, 1641–1658. [Google Scholar] [CrossRef]
- Bishop, P.D.; Nagel, C.W. Characterization of condensation product of malvidin 3,5-diglucoside and catechin. J. Sci. Food Agric. 1984, 32, 1022–1026. [Google Scholar] [CrossRef]
- Remy-Tanneau, S.; le Guerneve, C.; Meudec, E.; Cheynier, V. Characterization of a colorless anthocyanin-flavan-3-ol dimer containing both carbon–carbon and ether interflavanoid linkages by NMR and mass spectrometry. J. Sci. Food Agric. 2003, 51, 3592–3597. [Google Scholar]
- Burger, J.F.W.; Kolodziej, H.; Hemingway, R.W.; Steynberg, J.P.; Young, D.A.; Ferreira, D. Oligomeric flavanoids. Part W. base-catalyzed pyran rearrangements of procyanidin B-2, and evidence for the oxidative transformation of B- to A-type procyanidins. Tetrahedron 1990, 46, 5733–5740. [Google Scholar] [CrossRef]
- Kondo, K.; Kurihawa, M.; Fukuhara, K.; Tanaka, T.; Suzuki, T.; Miyata, N.; Toyoda, M. Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Tetrahedron Lett. 2000, 41, 485–488. [Google Scholar] [CrossRef]
- Kovac, V.; Alonso, E.; Bourzeix, M.; Revilla, E. Effect of several enological practices on the content of catechins and proanthocyanidins of red wines. J. Agric. Food Chem. 1992, 40, 1953–1957. [Google Scholar] [CrossRef]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwiatkowski, M.; Gawel, R.; Williams, P.; Waters, E.; Cheynier, V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual. Prefer. 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Cao, G.; Ou, B.; Prior, R.L. Anthocyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J. Agric. Food Chem. 2003, 51, 4889–4896. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Evaluation of bitterness and astringency of (+)-catechin and (-)-epicatechin in red wine and in model solution. J. Sens. Stud. 1997, 12, 25–37. [Google Scholar] [CrossRef]
- Thorngate, J.H.; Noble, A.C. Sensory evaluation of bitterness and astringency of 3R( -)-epicatechin and 3S( +)-catechin. J. Sci. Food Agric. 1995, 67, 531–535. [Google Scholar] [CrossRef]
- Lee, C.B.; Lawless, H.T. Time-course of astringent sensations. Chem. Senses 1991, 16, 225–238. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Cheynier, V.; Moutounet, M. Interactions of grape seed tannins with salivary proteins. J. Agric. Food Chem. 1999, 47, 42–47. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Del Llaudy, M.C.; Canals, R.; Canals, J.M.; Zamora, F. Influence of ripening stage and maceration length on the contribution of grape skins, seeds and stems to phenolic composition and astringency in wine-simulated macerations. Eur. Food Res. Technol. 2008, 226, 337–344. [Google Scholar] [CrossRef]
- Passos, C.P.; Cardoso, S.M.; Domingues, M.R.M.; Domingues, P.; Silva, C.M.; Coimbra, M.A. Evidence for galloylated type-A procyanidins in grape seeds. Food Chem. 2007, 105, 1457–1467. [Google Scholar] [CrossRef]
- Fernández, K.; Kennedy, J.A.; Agosin, E. Characterization of Vitis vinifera L. Cv. Carménère grape and wine proanthocyanidins. J. Agric. Food Chem. 2007, 55, 3675–3680. [Google Scholar]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Pati, S.; Losito, I.; Gambacorta, G.; La Notte, E.; Palmisano, F.; Zambonin, P.G. Simultaneous separation and identification of oligomeric procyanidins and anthocyanin-derived pigments in raw red wine by HPLC-UV-ESI-MSn. J. Mass Spectrom. 2006, 41, 861–871. [Google Scholar] [CrossRef]
- Haslam, E. In vino veritas: Oligomeric procyanidins and the ageing of red wines. Phytochemistry 1980, 19, 2577–2582. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier, V.; Moutounet, M. Analysis and characterization of wine condensed tannins precipitated by protein used as fining agent in enology. Am. J. Enol. Vitic. 1999, 50, 81–86. [Google Scholar]
- Maury, C.; Sarni-Manchado, P.; Lefèbvre, S.; Cheynier, V.; Moutounet, M. Influence of fining with different molecular weight gelatins on proanthocyanidin composition and perception of wines. Am. J. Enol. Vitic. 2001, 52, 140–145. [Google Scholar]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar]
- Vidal, S.; Cartalade, D.; Souquet, J.M.; Fulcrand, H.; Cheynier, V. Changes in proanthocyanidin chain length in winelike model solutions. J. Agric. Food Chem. 2002, 50, 2261–2266. [Google Scholar]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- Salas, E.; Le Guernevé, C.; Fulcrand, H.; Poncet-Legrand, C.; Cheynier, V. Structure determination and colour properties of a new directly linked flavanol–anthocyanin dimer. Tetrahedron Lett. 2004, 45, 8725–8729. [Google Scholar]
- Santos-Buelga, C.; Bravo-Haro, S.; Rivas-Gonzalo, J.C. Interactions between catechin and malvidin-3-monoglucoside in model solutions. Eur. Food Res..Technol. 1995, 201, 269–274. [Google Scholar]
- Es-Safi, N.-E.; Le Gueraevé, C.; Labarbe, B.; Fulerand, H.; Cheyuier, V.; Moutouaet, M. Structure of a new xanthyfium salt derivative. Tetrahedron Lett. 1999, 40, 5869–5872. [Google Scholar] [CrossRef]
- Jurd, L. Anthocyanidins and related compounds-XI Catechin-flavylium salt condensation reactions. Tetrahedron 1967, 23, 1057–1064. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Francia-Aricha, E.M.; De Pascual-Teresa, S.; Rivas-Gonzalo, J.C. Contribution to the identification of the pigments responsible for the browning of anthocyanin-flavanol solutions. Eur. Food Res. Technol. 1999, 209, 411–415. [Google Scholar] [CrossRef]
- Sun, W.; Miller, J.M. Tandem mass spectrometry of the B-type procyanidins in wine and B-type dehydrodicatechins in an autoxidation mixture of (+)-catechin and (−)-epicatechin. J. Mass Spectrom. 2003, 38, 438–446. [Google Scholar] [CrossRef]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Structural determination of colourless and yellow dimers resulting from (+)-catechin coupling catalyzed by grape polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar]
- Oszmianski, J.; Cheynier, V.; Moutounet, M. Iron-catalyzed oxidation of (+)-catechin in model systems. J. Agric. Food Chem. 1996, 44, 1712–1715. [Google Scholar]
- Sims, C.A.; Morris, J.R. Effects of acetaldehyde and tannins on the color and chemical age of red Muscadine (Vitis rotundifolia) wine. Am. J. Enol. Vitic. 1986, 37, 163–165. [Google Scholar]
- Saucier, C.; Little, D.; Glories, Y. First evidence of acetaldehyde-flavanol condensation products in red wine. Am. J. Enol. Vitic. 1997, 48, 370–373. [Google Scholar]
- Wildenradt, H.L.; Singleton, V.L. The production of aldehydes as a result of oxidation of polyphenolic compounds and its relation to wine aging. Am. J. Enol. Vitic. 1974, 25, 119–126. [Google Scholar]
- Rogerson, F.S.; De Freitas, V. Fortification spirit, a contributor to the aroma complexity of Porto. J. Food Sci. 2002, 67, 564–569. [Google Scholar]
- Bendz, G.; Martensson, O.; Nilsson, E. Studies of flavylium compounds. I. Some flavylium compounds and their properties. Arkiv Kemi 1967, 27, 67–77. [Google Scholar]
- Saucier, C.; Guerra, C.; Pianet, I.; Laguerre, M.; Glories, Y. (+)-Catechin-acetaldehyde condensation products in relation to wine-ageing. Phytochemistry 1997, 46, 229–234. [Google Scholar] [CrossRef]
- Rivas-Gonzalo, J.C.; Bravo-Haro, S.; Santos-Buelga, C. Detection of compounds rormed through the reaction of malvidin 3-monoglucoside and catechin in the presence of acetaldehyde. J, Agric. Food Chem. 1995, 43, 1444–1449. [Google Scholar]
- Saucier, C.; Bourgeois, G.; Vitry, C.; Roux, D.; Glories, Y. Characterization of (+)-catechin-acetaldehyde polymers: a model for colloidal state of wine polyphenols. J. Agric. Food Chem. 1997, 45, 1045–1049. [Google Scholar]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Competition between (+)-catechin and (-)-epicatechin in acetaldehyde-induced polymerization of flavanols. J. Agric. Food Chem. 1999, 47, 2088–2095. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the acetaldehyde-induced condensation of (-)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Interactions of oligomeric procyanidins in model wine solutions containing malvidin-3-glucoside and acetaldehyde. J. Sci. Food Agric. 1996, 70, 493–500. [Google Scholar] [CrossRef]
- Pissarra, J.; Mateus, N.; Rivas-Gonzalo, J.; Santos Buelga, C.; De Freitas, V. Reaction between malvidin 3-glucoside and (+)-catechin in model solutions containing different aldehydes. J. Food Sci. 2003, 68, 476–481. [Google Scholar] [CrossRef]
- Pissarra, J.; Lourenço, S.; González-Paramás, A.M.; Mateus, N.; Buelga, C.S.; Silva, A.M.S.; De Freitas, V. Isolation and structural characterization of new anthocyanin-alkyl-catechin pigments. Food Chem. 2005, 90, 81–87. [Google Scholar]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Study of the Reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem. 2000, 48, 5946–5954. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. New phenolic compounds formed by evolution of (+)-catechin and glyoxylic acid in hydroalcoholic solution and their implication in color changes of grape-derived foods. J. Agric. Food Chem. 2000, 48, 4233–4240. [Google Scholar] [CrossRef]
- Fulcrand, H.; Cheynier, V.; Oszmanski, J.; Moutounet, M. An oxidized tartaric acid residue as a new bridge potentially competing with acetaldehyde in flavan-3-ol condensation. Phytochemistry 1997, 46, 223–227. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Technol. 2000, 35, 63–74. [Google Scholar] [CrossRef]
- Drinkine, J.; Glories, Y.; Saucier, C. (+)-Catechin-aldehyde condensations: Competition between acetaldehyde and glyoxylic acid. J. Agric. Food Chem. 2005, 53, 7552–7558. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Honda, T.; Kuwano, H.; Saito, N.; Terahara, N.; Timberlake, C.F. Identification of an anthocyanin occurring in some red wines. Phytochemistry 1997, 44, 1375–1382. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Francia-Aricha, E.; Rivas-Gonzalo, J.C. Formation of anthocyanin-derived pigments in experimental red wines. Food Sci. Technol. Int. 1999, 5, 347–352. [Google Scholar] [CrossRef]
- Francia-Aricha, E.M.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; De Freitas, V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric.Food Chem. 2003, 51, 1919–1923. [Google Scholar]
- De Freitas, V.A.P.; Mateus, N. Structural changes of anthocyanins during red wine aging: Portisins: A new class of blue anthocyanin-derived pigments. ACS Symp. Ser. 2004, 886, 160–178. [Google Scholar]
- Tao, J.; Dykes, S.; Kilmartin, P.A. Effect of SO2 concentration on polyphenol development during red wine micro-oxygenation. J. Agric. Food Chem. 2007, 55, 6104–6109. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, F.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. Chemical Synthesis of Proanthocyanidins in Vitro and Their Reactions in Aging Wines. Molecules 2008, 13, 3007-3032. https://doi.org/10.3390/molecules13123007
He F, Pan Q-H, Shi Y, Duan C-Q. Chemical Synthesis of Proanthocyanidins in Vitro and Their Reactions in Aging Wines. Molecules. 2008; 13(12):3007-3032. https://doi.org/10.3390/molecules13123007
Chicago/Turabian StyleHe, Fei, Qiu-Hong Pan, Ying Shi, and Chang-Qing Duan. 2008. "Chemical Synthesis of Proanthocyanidins in Vitro and Their Reactions in Aging Wines" Molecules 13, no. 12: 3007-3032. https://doi.org/10.3390/molecules13123007







