Brominated Thiophenes as Precursors in the Preparation of Brominated and Arylated Anthraquinones
Abstract
:1. Introduction
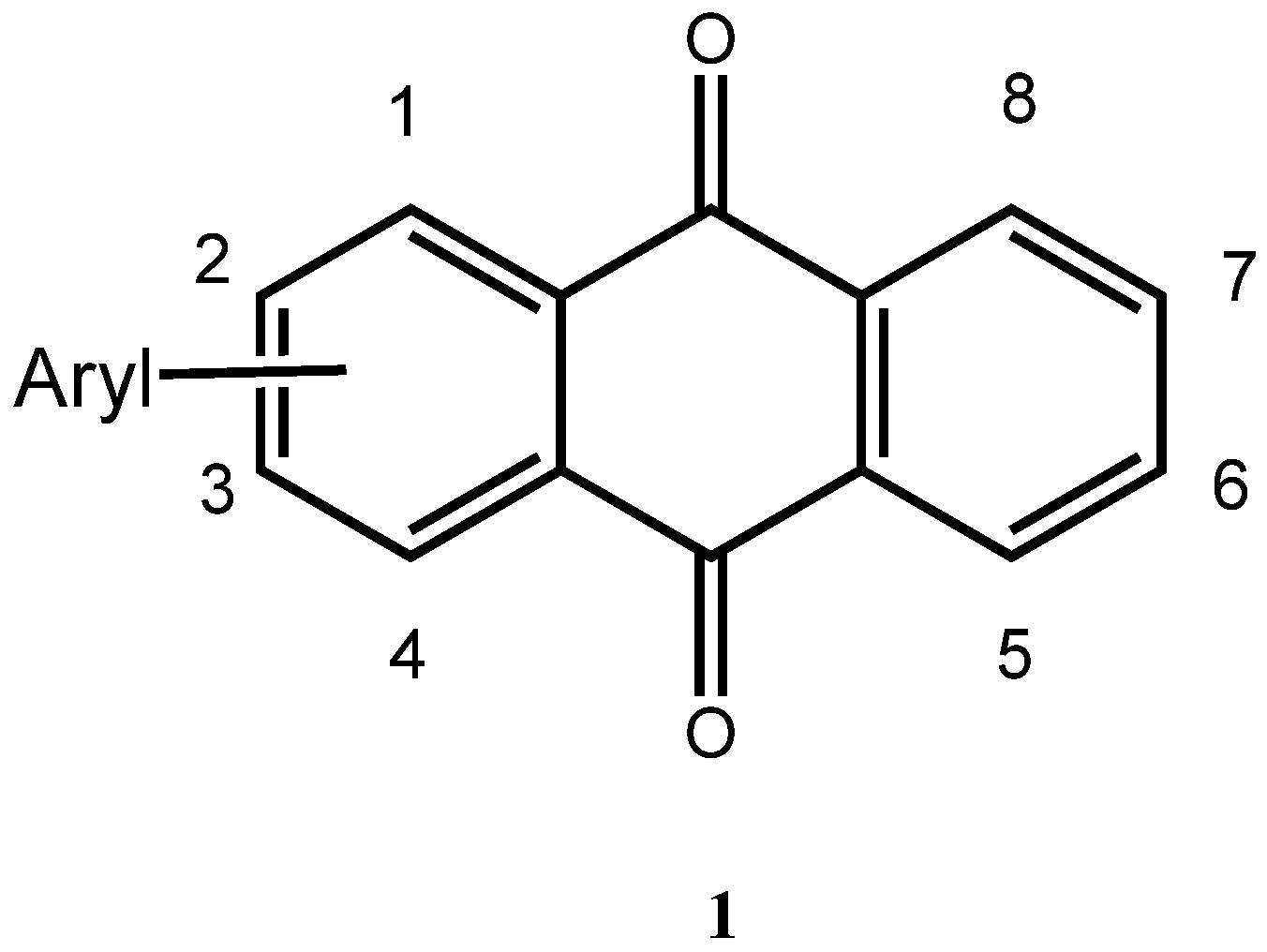
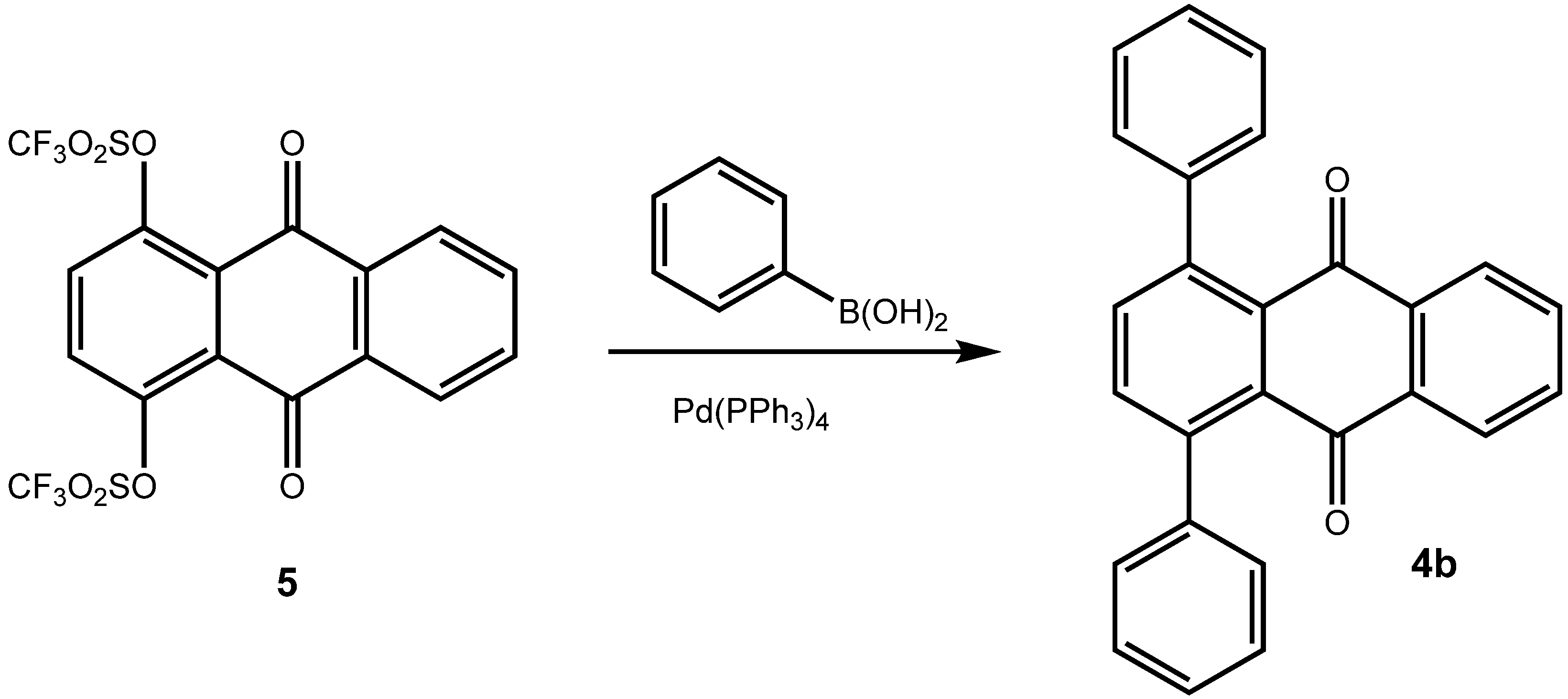
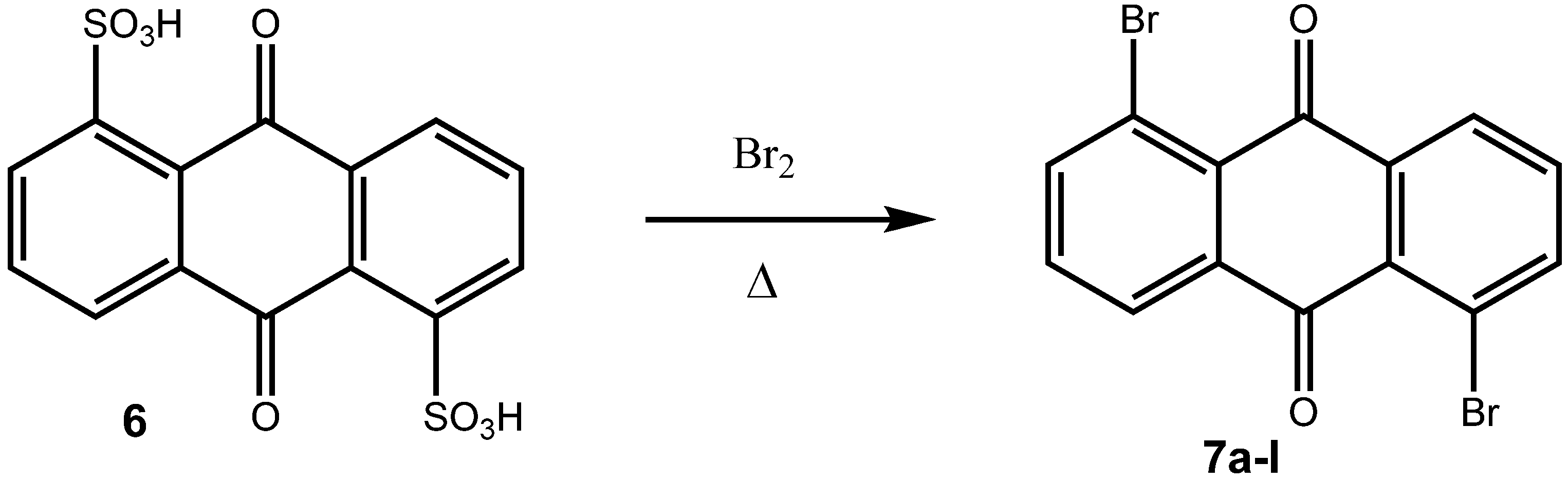

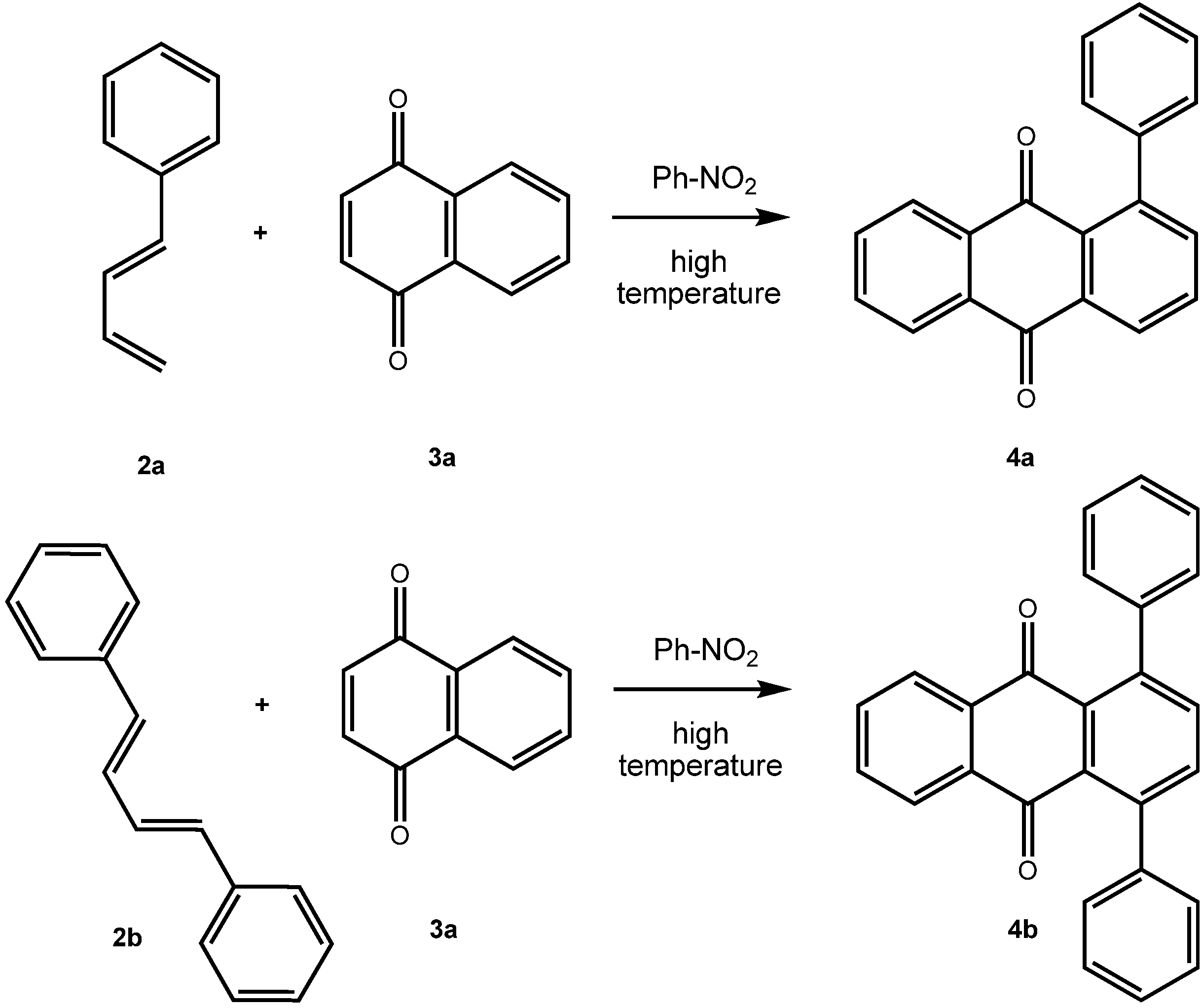
2. Results and Discussion
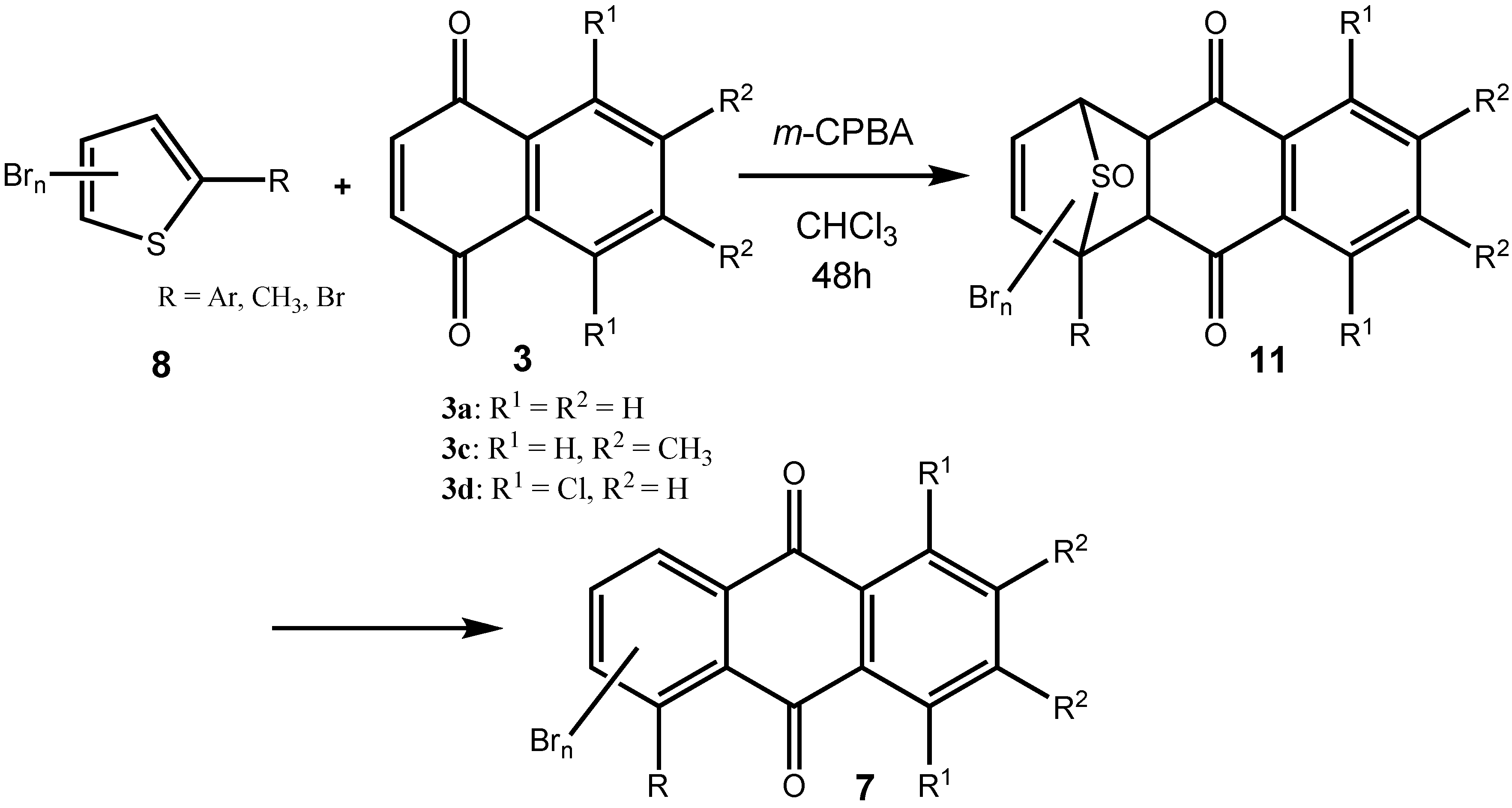
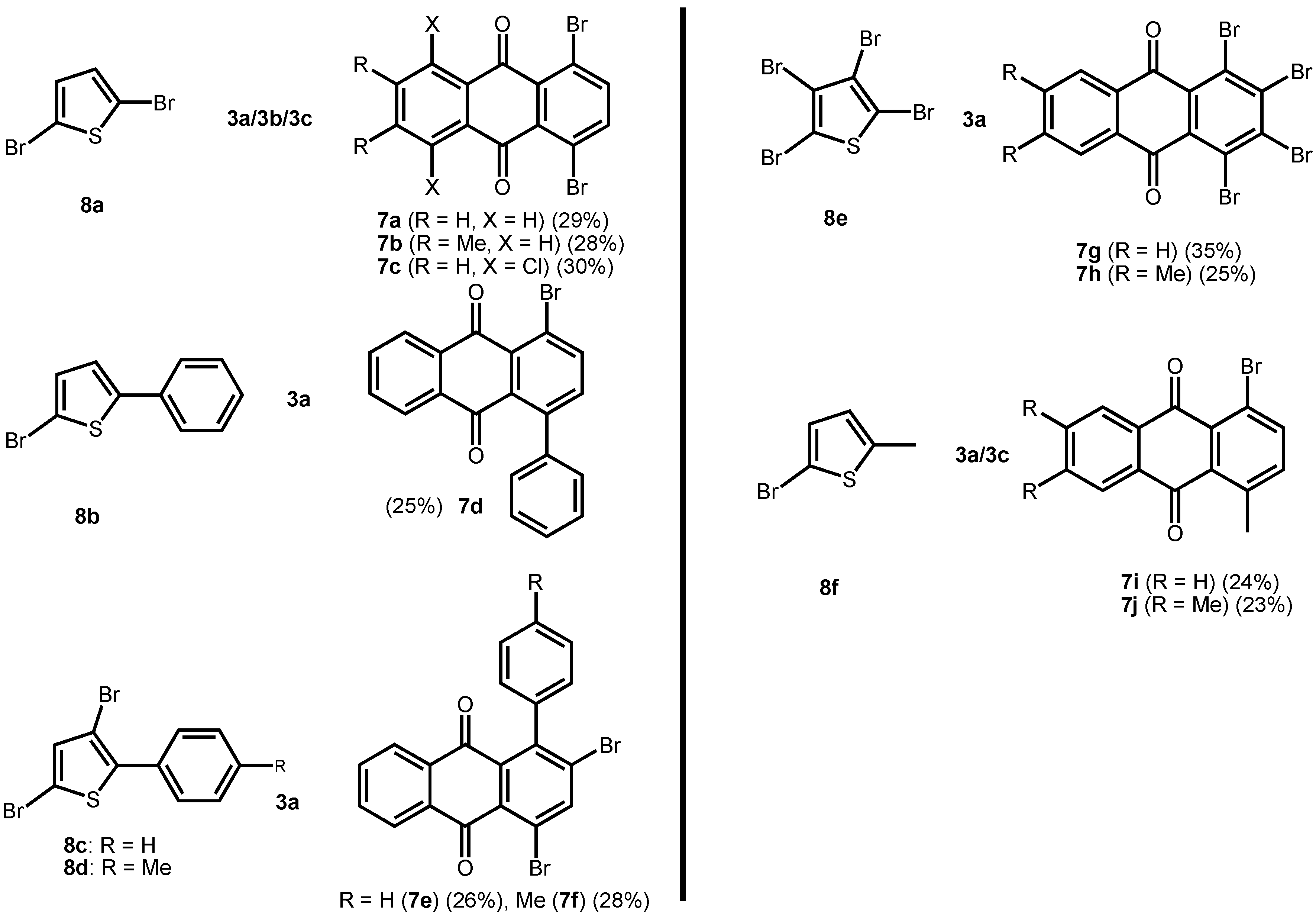 |
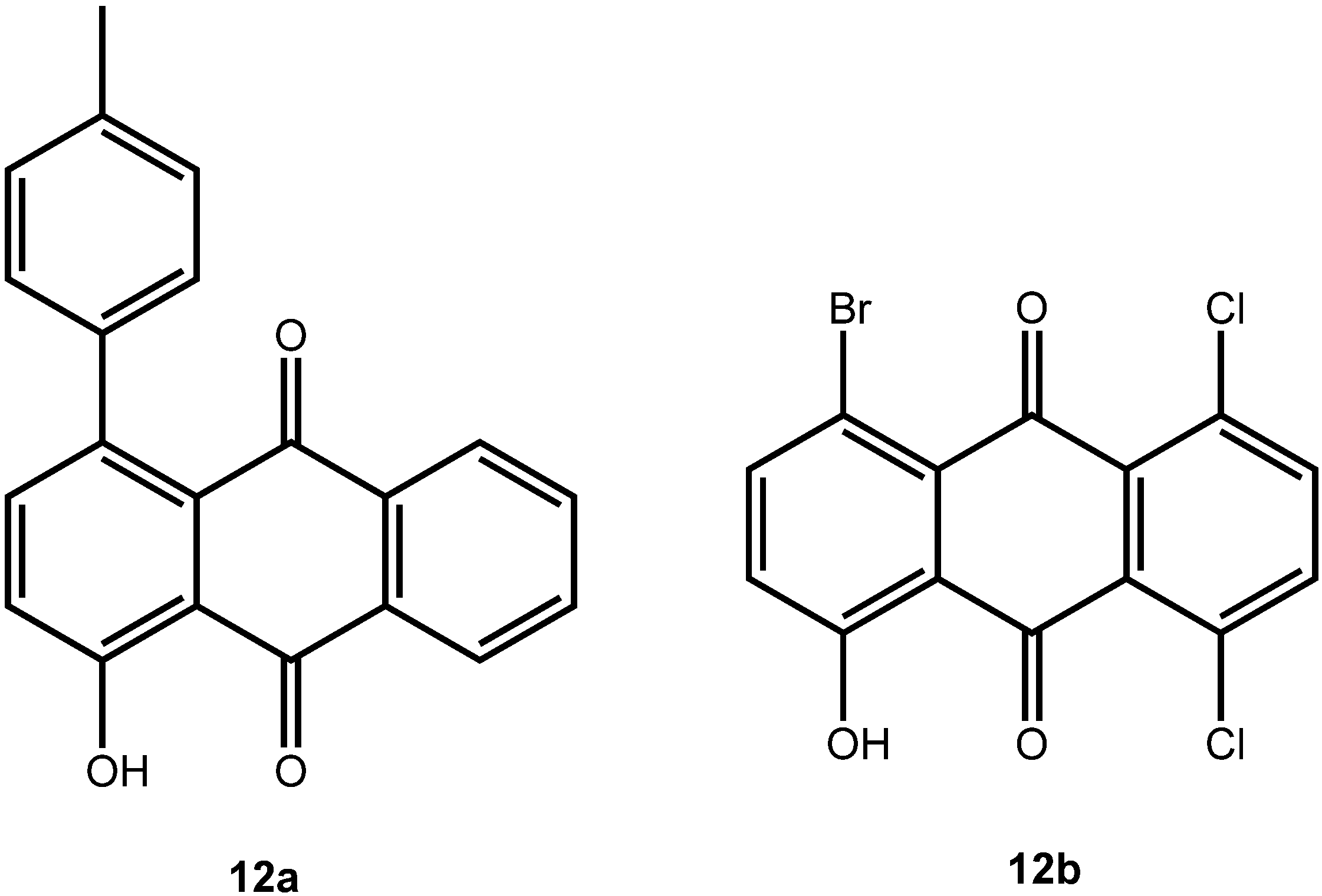
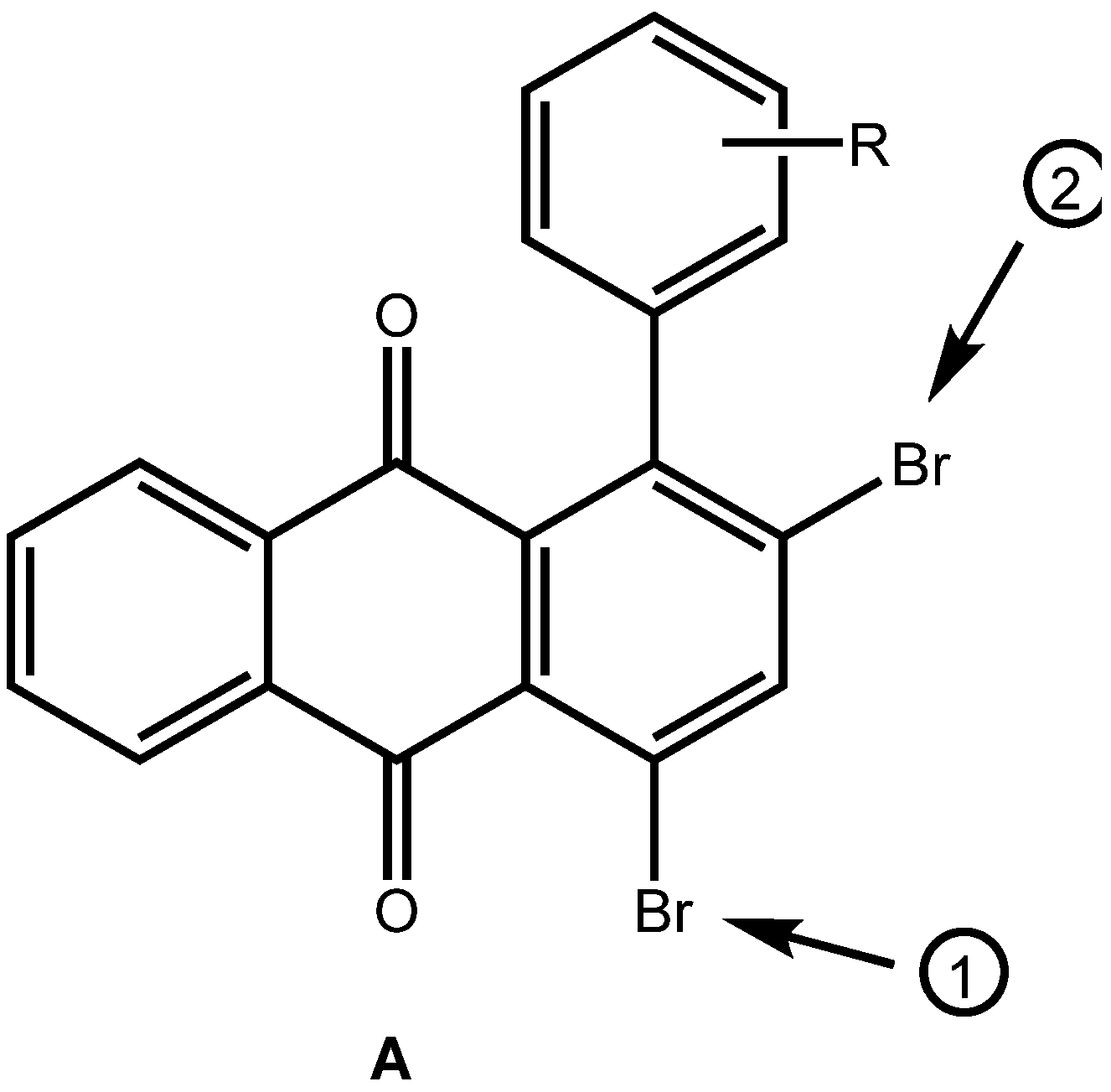
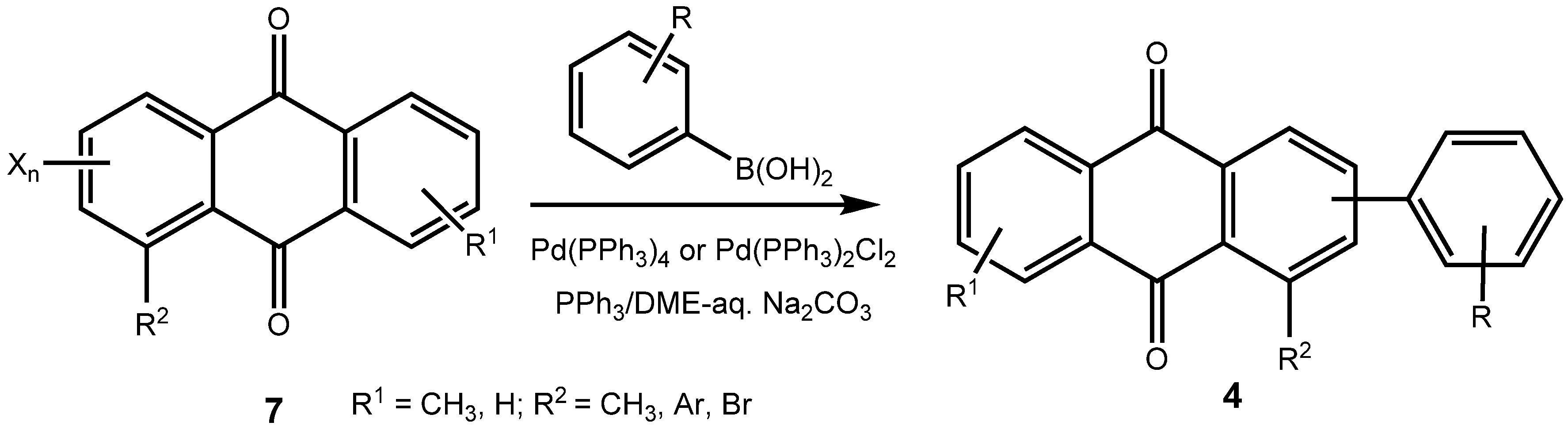
 |
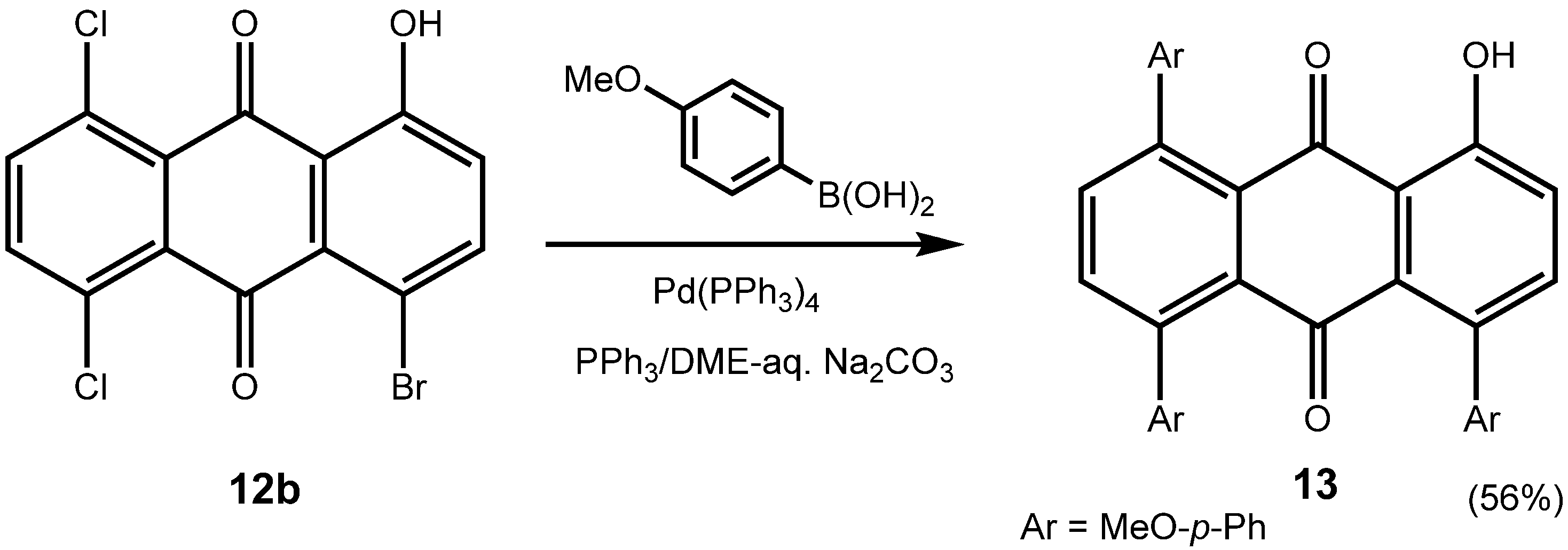
3. Experimental Section
General
Selected data of other bromoanthraquinones
Selected data for other arylated anthraquinones
4. Conclusions
Acknowledgements
References and Notes
- Josien, J.M.; Fuson, N. The infrared study of the valence bond C=O in polycyclic compounds. Bull. Soc. Chim. Fr. 1952, 389–397. [Google Scholar]
- Gautrot, J.E.; Hodge, P.; Cupertino, D.; Helliwell, M. Experimental evidence for carbonyl-π electron cloud interactions. New J. Chem. 2006, 30, 1801–1807. [Google Scholar] [CrossRef]
- Showalter, H.D.H.; Berman, E.M.; Johnson, J.L.; Hunter, W.E. A facile synthesis of functionalized 9,10-anthracenediones via tosylate and triflatephenolic activation. Tetrahedron Lett. 1985, 26, 157–160. [Google Scholar] [CrossRef]
- Shcheglova, N.A.; Shigorin, D.N.; Dokunikhin, N.S. Characteristics of luminescence and absorption spectra of 1- and 2-alkyl- and arylanthraquinones in solutions. II. Luminescence spectra at 77 K. Zh. Fiz. Khim. 1966, 40, 1048–1055, [Chem. Abstr. 1966, 65, 9958b]. [Google Scholar]
- Shcheglova, N.A.; Shigorin, D.N.; Dokunikhin, N.S. Spectra of luminescence and absorption of α - and β -alkyl and aryl derivatives of anthraquinone in solutions. I. Absorption spectra of α - and β -alkyl and aryl derivatives of anthraquinone. Zh. Fiz. Khim. 1965, 39, 3039–3043, [Chem. Abstr. 1965, 64, 12058f]. [Google Scholar]
- Orser, D.A. (General Electric Company, Schenectady, N.Y., USA) Light-modulating medium for image projection apparatus. US Pat. 3764549 9 Oct. 1973. [Chem. Abstr. 1973, 79, 141431j]. [Google Scholar]
- Weizmann, C.; Bergmann, E.; Haskelberg, L. Phenylated phthalic acids and anthracene derivatives. J. Chem. Soc. 1939, 391–397. [Google Scholar] [CrossRef]
- Bergmann, E.; Haskelberg, L.; Bergmann, F. Successive diene addition and dehydrogenation in nitrobenzene solution without isolation of the hydroaromatic intermediate. J. Org. Chem. 1942, 7, 303–306. [Google Scholar] [CrossRef]
- Muschik, G.M.; Tomaszewski, J.E.; Sato, R.I. Synthesis of the 1-,2-,3-,4-hydroxy isomers of benz[a]anthracene-7,12-dione, benz[a]anthracene, and 7,12-dimethylbenz[a]anthracene. J. Org. Chem. 1979, 44, 2150–2153. [Google Scholar] [CrossRef]
- Puchkov, V.A. Synthesis of 1-phenylanthraquinone. Zh. Vsesoy. Khim. Obshch. im. D. I. Mendeleeva 1961, 6, 238–239, [Chem. Abstr. 1961, 55, 19877g]. [Google Scholar]
- Battegay, M.; Claudin, J. Dibromoanthraquinones. Bull. Soc. Chim. Fr. 1921, 29, 1017–1027. [Google Scholar]
- Li, Y.Q.; Thiemann, T.; Sawada, T.; Tashiro, M. Novel crown ethers by oxidative cycloaddition of thiopheno crown ethers. J. Chem. Soc., Perkin Trans. 1 1994, 2323–2329. [Google Scholar]
- Thiemann, T.; Li, Y.Q.; Mataka, S.; Tashiro, M. Intramolecular oxidative cycloaddition of thiophenes. J. Chem. Res. (S) 1995, 384–384, (M) 1995, 2364-2379. [Google Scholar]
- Thiemann, T.; Li, Y.Q.; Thiemann, C.; Sawada, T.; Ohira, D.; Tashiro, M.; Mataka, S. Oxidative cycloaddition of structures with multi core thiophenes. Heterocycles 2000, 1215–1230. [Google Scholar]
- Li, Y.Q.; Matsuda, M.; Thiemann, T.; Sawada, T.; Mataka, S. Lewis acid catalysed oxidative cycloaddition of thiophenes. Synlett. 1996, 461–464. [Google Scholar]
- Li, Y.Q.; Thiemann, T.; Sawada, T.; Mataka, S.; Tashiro, M. Lewis acid catalysis in the oxidative cycloaddition of thiophenes. J. Org. Chem. 1997, 62, 7926–7936. [Google Scholar] [CrossRef]
- Li, Y.Q.; Thiemann, T.; Mimura, K.; Sawada, T.; Mataka, S.; Tashiro, M. Oxidative cycloaddition of thiophenophanes – [n](2,5)-parathiophenophane (n=8,10-12.14). [8](2,4)metathiophenophane and [2.2](2,5)parametathiophenophane. Eur. J. Org. Chem. 1998, 1841–1850. [Google Scholar]
- for reviews, see: Thiemann, T.; Dongol, K.G. Thiophene-S-oxides. J. Chem. Res. (S) 2002, 303–308, (M) 2002, 701-708 and ref.18. [Google Scholar] [CrossRef]
- Thiemann, T.; Walton, D.J.; Brett, A.O.; Iniesta, J.; Marken, F.; Li, Y.Q. The chemistry of thiophene-S-oxides and related compounds. ARKIVOC 2009, Vol. ix, 96–113. [Google Scholar]
- Exceptions are strained thiophenes: Nakayama, J.; Kuroda, K. Synthesis and reactivities of a highly strained thiophene with two fused four-membered rings, 1,2,4,5-tetahydrodicyclobuta[b,d]thiophene. J. Am. Chem. Soc. 1993, 115, 4612–4617, and ref. 21, 22. [Google Scholar] [CrossRef]
- Thiophenes with very effective electron-donor substituents: Barker, J.M.; Huddleston, P.R.; Shutler, S.W. Preparation and reactions of 2,5-dimethoxythiophene. J. Chem. Soc., Perkin Trans. 1 1975, 2483–2484. [Google Scholar]
- Wynberg, H.; Helder, R. One-step ring enlargement of a thiophene to a thiepin. Tetrahedron Lett. 1972, 3647–3650. [Google Scholar] [CrossRef]
- This is underscored by theoretical calculations, see, cf.: Jursic, B.S.; Coupe, D. AM1 semiempirical searching for suitable thiophene derivatives that will enable thiophenes to act as a diene in the Diels-Alder reactions. J. Heterocycl. Chem. 1995, 32, 483–489. [Google Scholar]
- Furukawa, N.; Zhang, S.; Sato, S.; Higaki, M. Simple procedure for the synthesis of 2,5-bis(silylated) thiophene S-oxides with m-chloroperbenzoic acid in the presence of BF3(Et2O). Heterocycles 1997, 44, 61–66. [Google Scholar] [CrossRef]
- Nakayama, J.; Nagasawa, H.; Sugihara, Y.; Ishii, A. Synthesis, isolation, and full characterization of the parent thiophene 1,1-dioxide. J. Am. Chem. Soc. 1997, 119, 9077–9078. [Google Scholar] [CrossRef]
- Furukawa, N.; Zhang, S.-Z.; Horn, E.; Takahashi, S.; Sato, S.; Yokoyama, M.; Yamaguchi, K. X-ray crystallographic analysis of 2,5-bis(diphenylmethylsilyl)thiophenemonooxide and the Diels-Alder reaction of thiophenemonooxide with dienophiles. Heterocycles 1998, 47, 793–809. [Google Scholar] [CrossRef]
- Pouzet, P.; Erdelmeier, I.; Ginderow, D.; Mornon, J.-P.; Dansette, P.M.; Mansuy, D. Thiophene 1-oxides. V. comparison of the crystal structures and thiophene ring aromaticity of 2,5-diphenylthiophene, its sulfoxide and sulfone. J. Heterocycl. Chem. 1997, 34, 1567–1574. [Google Scholar] [CrossRef]
- Pouzet, P.; Erdelmeier, I.; Ginderow, D.; Mornon, J.-P.; Dansette, P.M.; Mansuy, D. Thiophene S-oxides: convenient preparation, first complete structural characterization and unexpected dimerization of one of them, 2,5-diphenylthiophene 1-oxide. J. Chem. Soc., Chem. Commun. 1995, 473–474. [Google Scholar]
- Torssell, K. Diels-Alder reactions of thiophene oxides generated in situ. Acta Chem. Scand., Ser. B 1976, 30, 353–357. [Google Scholar] [CrossRef]
- Thiemann, T.; Sa e Melo, M.L.; Campos Neves, A.S.; Li, Y.Q.; Mataka, S.; Tashiro, M.; Geiβler, U.; Walton, D. Preparation and electrooxidative SO-extrusion of halogenated 7-thiabicyclo[2.2.1]heptene 7-oxides. J. Chem. Res. (S) 1998, 346–347. [Google Scholar]
- Raasch, M.S. Annulations with tetrachlorothiophene 1,1-dioxide. J. Org. Chem. 1980, 45, 856–867. [Google Scholar] [CrossRef]
- Raasch, M.S. Addition-rearrangement reactions of halogenated thiophene dioxides with furans. J. Org. Chem. 1980, 45, 867–870. [Google Scholar] [CrossRef]
- Hamon, D.P.G.; Spurr, P.R. A simple construction of the iceane skeleton via an intramolecular Diels-Alder reaction. J. Chem. Soc., Chem. Commun. 1982, 372–373. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry; Georg Thieme Verlag: Stuttgart, Germany, 1997; p. 246. [Google Scholar]
- Wraight, E.S. Some Newer Phys. Methods Struct. Chem., Proc. Symp. 1967, 67–67, [Chem. Abstr. 1969, 70, 62337u].
- Yoshida, Z.; Takabayashi, F. Electronic spectra of monosubstituted anthraquinones and solvent effects. Tetrahedron 1968, 24, 933–943. [Google Scholar] [CrossRef]
- Peters, R.H.; Sumners, H. Spectra of anthraquinone derivatives. J. Chem. Soc. 1953, 2101–2110. [Google Scholar] [CrossRef]
- cf., Ertas, E.; Ozturk, T. A new reaction of P4S10 and Lawesson's reagent; a new method for the synthesis of dithieno[3,2-b;2',3'-d]thiophenes. Tetrahedron Lett. 2004, 45, 3405–3407. [Google Scholar] [CrossRef]
- see also: Goldberg, Y.; Alper, H. Electrophilic halogenation of aromatics and heteroaromatics with N-halosuccinimides in a solid/liquid system using an H+ ion exchanger or ultrasonic irradiation. J. Mol. Cat. 1994, 88, 377–383. [Google Scholar] [CrossRef]
- Gjoes, N.; Gronowitz, S. Substitution reactions of 2-phenylthiophene. Acta Chem. Scand. 1972, 26, 1851–1859. [Google Scholar] [CrossRef]
- This reaction has been reported to proceed under YbCl3 catalysis: Fang, X.; Warner, B.P.; Watkin, J.G. Ytterbium trichloride-catalyzed Diels-Alder reactions of un-activated dienes. Synth. Commun. 2000, 30, 2669–2676. [Google Scholar] We have found that it proceeds also in the presence of EuCl3.
- Here, we have used a biphasic system of 4N aq. NaOH and ether under ultrasonication. The enolisation has been reported to also go very well in the presence of triethylamine (Et3N) or in the presence of acids such as HCl: Fernandez, M.P.; Gonzalez, B.; Pardo, M.; Soto, J.L. 1,3-Dipolar cycloaddition of nitrile oxides with Diels-Alder adducts of p-benzoquinone and 1,4-naphthoquinone. Anal. Quim. 1994, 90, 477–482. [Google Scholar] (for Et3N), also see ref. [43]
- Kotha, S.; Manivannan, E. Synthesis of functionalized cis-syn-cis triquinanes. Ind. J. Chem., Sect. B 2002, 41B, 808–811, (for HCl). [Google Scholar]
- We used Ag2O in benzene as the oxidation agent as described for the oxidation of other hydroquinones to quinones. The reaction gives quantitative yields of 6,7-dimethyl-5,8-dihydro-1,4-naphthoquinone, when carried out at rt. Specifically for the transformation of 6,7-dimethyl-5,8-dihydronaphthalene-1,4-diol to 6,7-dimethyl-5,8-dihydro-1,4-naphthoquinone, the use of MnO2 in acetone has been described, also: ref. [43].
- cf., Fuchibe, K.; Akiyama, T. Low-valent niobium-mediated double activation of C-F/C-H bonds: fluorene synthesis from o-arylated alpha,alpha,alpha-trifluorotoluene derivatives. J. Am. Chem. Soc. 2006, 128, 1434–1435. [Google Scholar] [CrossRef]
- Kondo, M.; Murata, M.; Nishihara, H.; Nishibori, E.; Aoyagi, S.; Yoshida, M.; Kinoshita, Y.; Sakata, M. Guest-induced instant and reversible crystal-to-crystal transformation of 1,4-bis(ferrocenylethynyl)anthraquinone. Angew. Chem. Int. Ed. Engl. 2006, 45, 5461–5464. [Google Scholar] [CrossRef]
- Hofmann, A. Substituted benzoylbenzoic acids. Monatsh. Chem. 1915, 36, 805–824. [Google Scholar] [CrossRef]
- Castle, R.N.; Kudo, H.; Lee, M.L. The synthesis of some monomethylanthracenamines. Coll. Czech Chem. Commun. 1991, 56, 2269–2277. [Google Scholar] [CrossRef]
- Mizuno, H.; Kubota, S. (Seiko Epson Corp.), Method of distinguishing right from left contact lenses by coloring. JP Pat. 04011214 1992. [Chem. Abstr. 1992, 116, 221636]. [Google Scholar]
- Ganushchak, N.I.; Dombrovskii, A.V.; Vislobitskaya, O.A. Syntheses based on Diels-Alder condensations. I. 1-Methyl-4-arylanthraquinones. Zh. Obshch. Khim. 1963, 33, 2532–2534. [Google Scholar]
- Sample Availability: Samples of the compounds 4b-4f, 4m are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thiemann, T.; Tanaka, Y.; Iniesta, J. Brominated Thiophenes as Precursors in the Preparation of Brominated and Arylated Anthraquinones. Molecules 2009, 14, 1013-1031. https://doi.org/10.3390/molecules14031013
Thiemann T, Tanaka Y, Iniesta J. Brominated Thiophenes as Precursors in the Preparation of Brominated and Arylated Anthraquinones. Molecules. 2009; 14(3):1013-1031. https://doi.org/10.3390/molecules14031013
Chicago/Turabian StyleThiemann, Thies, Yasuko Tanaka, and Jesus Iniesta. 2009. "Brominated Thiophenes as Precursors in the Preparation of Brominated and Arylated Anthraquinones" Molecules 14, no. 3: 1013-1031. https://doi.org/10.3390/molecules14031013