Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves
Abstract
:Introduction

Results and Discussion
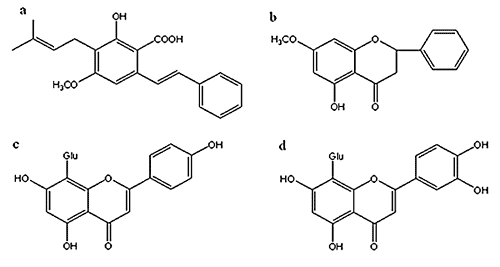
Amounts of total flavonoids and contents of main components
| Sample | Contents (mg/g extract) | ||||
|---|---|---|---|---|---|
| Flavonoids | Cajaninstilbene acid | Pinostrobin | Vitexin | Orientin | |
| Aqueous extracts | 146.32±3.28 | 0.545±0.12 | 0.89±0.68 | 1.25±0.21 | 1.07±0.32 |
| Ethanol extracts | 293.45±3.12 | 32.76±1.67 | 11.85±1.41 | 6.21±0.35 | 6.13±2.21 |
| Petroleum ether fraction | 151.29±1.55 | 69.93±1.39 | 30.29±2.09 | 0.253±0.07 | 0.122±0.09 |
| Ethyl acetate fraction | 384.43±1.58 | 37.795±2.13 | 12.339±2.53 | 21.03±2.23 | 18.82±1.13 |
| n-Butanol fraction | 211.37±2.19 | - | - | 5.697±3.26 | 8.331±1.08 |
| Water fraction | 52.43±1.64 | - | - | - | 4.383±0.24 |

Antioxidant activities
| Sample | DPPH radical scavenging assay IC50 (µg/mL) | β-carotene-linoleic acid testIC50 (µg/mL) |
|---|---|---|
| Aqueous extracts | 404.91* | 475.26† |
| Ethanol extracts | 242.01* | 256.88† |
| Petroleum ether fraction | 553.41* | 581.99† |
| Ethyl acetate fraction | 194.98* | 213.89† |
| n-Butanol fraction | 382.23* | 410.47† |
| Water fraction | 2388.31* | 2663.91† |
| Cajaninstilbene acid | 302.12* | 321.53† |
| Pinostrobin | >500* | >500† |
| Vitexin | >500* | >500† |
| Orientin | 316.21* | 444.61† |
| Ascorbic acid | 201.29 | - |
| BHT | - | 195.74 |
Experimental
General
Plant material
Preparation of the ethanol and aqueous extracts
Separation of target components
HPLC analysis
Determination of total flavonoids
Determination of antioxidant activity
Statistical analysis
Acknowledgements
References
- Chakraborty, S.K.; Kumbhar. B.K.; Sarkar, B.C. Process parameter optimization for instant pigeonpea dhal using response surface methodology. J. Food. Eng. 2007, 81, 171–178. [Google Scholar]
- Salunkhe, D.K.; Chavan, J.K.; Kadam, S.S. Pigeonpea as an important food source. Crit. Rev. Food Sci. 1986, 23, 103–145. [Google Scholar] [CrossRef]
- Fu, Y.J.; Zu, Y.G.; Liu, W.; Efferth, T.; Zhang, N.J.; Liu, X.N.; Kong, Y. Optimization of luteolin separation from pigeonpea [Cajanus cajan (L.) Millsp.] leaves by macroporous resins. J. Chromatogr. A 2006, 1137, 145–152. [Google Scholar]
- Fu, Y.J.; Zu, Y.G.; Liu, W.; Hou, C.L.; Chen, L.Y.; Li, S.M.; Shi, X.G.; Tong, M.H. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J. Chromatogr. A 1139, 206–213. [Google Scholar]
- Amalraj, T.; Ignacimuthu, S. Evaluation of the hypoglycaemic effect of Cajanus cajan (seeds) in mice. Indian J. Exp. Biol. 1998, 36, 1032–1033. [Google Scholar]
- Grover, J.K.; Yadav, S.; Vats, V.J. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar]
- Duke, J.A.; Vasquez, R. Amazonian Ethnobotanical Dictionary; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Abbiw, D.K. Useful Plants of Ghana; Richmond Intermediate Technology Publications and Royal Botanic Gardens: Kew, London, UK, 1990. [Google Scholar]
- Tang, Y.; Wang, B.; Zhou, X.J. Effect of external application of herbal cajani preparation on the fibronection content during healing process of open wound. J. Guangzhou U. Tradit. Chin. Med. 1999, 16, 302–304. [Google Scholar]
- Aiyeloja, A.A.; Bello, O.A. Ethnobotanical potentials of common herbs in Nigeria: A case study of Enugu state. Educat. Res. Rev. 2006, 1, 16–22. [Google Scholar]
- Chen, D.H.; Li, H.Y; Lin, H. Studies on chemical constituents in pigeonpea leaves. Chin. Tradit. Herb Drugs 1985, 16, 134–136. [Google Scholar]
- Li, Z.H.; Zhou, C.H.; Zhang, J.Y. The present status of study and utilization of pigeonpea in China and its prospects. Forest Res. 2001, 14, 674–681. [Google Scholar]
- Luo, Q.F.; Sun, L.; Si, J.Y.; Chen, D.H. Hypocholesterolemic effect of stilbenes containing extract-fraction from Cajanus cajan L. on diet-induced hypercholesterolemia in mice. Phytomedicine 2008, 15, 932–939. [Google Scholar]
- Huang, G.Y.; Liao, X.Z.; Liao, H.F.; Deng, S.J.; Tan, Y.H.; Zhou, J.Y. Studies on water-soluble extracts from Cajanus cajan leaf against hypoxic-ischemic brain damage. Tradit. Chin. Drug Res. Clin. Pharmacol 2006, 17, 172–174. [Google Scholar]
- Kundu, R.; Dasgupta, S.; Biswas, A.; Bhattacharya, A.; Pal, B.C.; Bandyopadhyay, D.; Bhattacharya, S.; Bhattacharya, S. Cajanus cajan Linn. (Leguminosae) prevents alcohol-induced rat liver damage and augments cytoprotective function. J. Ethnopharmacol. 2008, 118, 440–447. [Google Scholar] [CrossRef]
- Duker-Eshun, G.; Jaroszewski, J.W; Asomaning, W.A.; Oppong-Boachie, F; Christensen, S.B. Antiplasmodial constituents of Cajanus cajan. Phytother Res. 2004, 18, 128–130. [Google Scholar]
- Zu, Y.G.; Fu, Y.J.; Liu, W.; Hou, C.L.; Kong, Y. Simultaneous determination of four flavonoids in pigeonpea [Cajanus cajan (L.) Millsp.] leaves using RP-LC-DAD. Chromatographia 2006, 63, 499–505. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Yang, J.; Chen, D.H.; Sun, L. Effects of the stilbene extracts from Cajanus cajan L. on ovariectomy-induced bone loss in rats. Acta Pharm. Sin. 2007, 42, 562–565. [Google Scholar]
- Gülçin, İ.; Oktay, M.; Küfrevioğlu, Ö. İ.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L). Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef]
- Madsen, H.L.; Bertelsen, G. Spices as antioxidants. Trends Food Sci. Tech. 1995, 6, 271–277. [Google Scholar]
- Ito, N.; Hirose, M.; Fukushima, H.; Tsuda, T.; Shirai, T.; Tatenatsu, M. Studies on antioxidants: Their carcinogenic and modifying effects on chemical carcinogens. Food Chem. Toxicol. 1986, 24, 1071–1092. [Google Scholar]
- Stich, H.F. The beneficial and hazardous effects of simple phenolic compounds. Mutat. Res. 1991, 259, 307–324. [Google Scholar]
- Jia, Z.B; Tao, F.; Guo, L.; Tao, G.J.; Ding, X.L. Antioxidant properties of extracts from juemingzi (Cassia tora L.) evaluated in vitro. LWT 2007, 40, 1072–1077. [Google Scholar] [CrossRef]
- Baratto, M.C.; Tattini, M.; Galardi, C.; Pinelli, P.; Romani, A.; Visiolid, F.; Basosi, R.; Poqni, R. Antioxidant activity of Galloyl quinic derivatives isolated from Pistacia lentiscus leaves. Free Radical Res. 2003, 37, 405–412. [Google Scholar] [CrossRef]
- Katalynic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Larson, R.A. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Liu, H.Y.; Qiu, N.X.; Ding, H.H.; Yao, R.Q. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Oboh, G. Antioxidant properties of some commonly consumed and underutilized tropical legumes. Eur. Food Res. Technol. 2006, 224, 61–65. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant activity of Caesalpinia digyna root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, X.P.; Jin, Z.Y.; Tian, Y.; Song, H.L. Free radical and reactive oxygen species scavenging activities of peanut skins extract. Food Chem. 2007, 104, 242–250. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Cherdshewasart, W.; Sutjit, W. Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine 2008, 15, 38–43. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van den Berg, D.J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radical Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Jed, W.; Fahey; Katherine, K. Stephenson Pinostrobin from Honey and Thai Ginger (Boesenbergia pandurata): A Potent Flavonoid Inducer of Mammalian Phase 2 Chemoprotective and Antioxidant Enzymes. J. Agric. Food Chem. 2002, 50, 7472–7476. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Dahiya, J.S.; Garratt, P.J.; Strange, R.N. Two novel stilbene-2-carboxylic acid phytoalexins from Cajanus cajan. Phytochemistry 1982, 21, 2935–2938. [Google Scholar]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, J.; Fan, G..; Wu, Y. Isolation and purification of flavonoid glycosides from Trollius lebebouri using high-speed counter-counter chromatography by stepwise increasing the flow-rate of the mobile phase. J. Chromatogr. A 2005, 1092, 216–221. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Moghaddam, P.R.; Barl, B.; Weil, J. A Free-radical scavenging capacity and antioxidant activity of selected plant species from Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agr. Food Chem. 1994, 42, 629–632. [Google Scholar]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidant. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, N.; Fu, K.; Fu, Y.-J.; Zu, Y.-G.; Chang, F.-R.; Chen, Y.-H.; Liu, X.-L.; Kong, Y.; Liu, W.; Gu, C.-B. Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves. Molecules 2009, 14, 1032-1043. https://doi.org/10.3390/molecules14031032
Wu N, Fu K, Fu Y-J, Zu Y-G, Chang F-R, Chen Y-H, Liu X-L, Kong Y, Liu W, Gu C-B. Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves. Molecules. 2009; 14(3):1032-1043. https://doi.org/10.3390/molecules14031032
Chicago/Turabian StyleWu, Nan, Kuang Fu, Yu-Jie Fu, Yuan-Gang Zu, Fang-Rong Chang, Yung-Husan Chen, Xiao-Lei Liu, Yu Kong, Wei Liu, and Cheng-Bo Gu. 2009. "Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves" Molecules 14, no. 3: 1032-1043. https://doi.org/10.3390/molecules14031032