1. Introduction
Medicinal plants are an important source of natural compounds with biological properties, including antimicrobial effects. Due to the occurrence of resistance to antimicrobials and the incidence of infectious diseases, there is a need to search for new antimicrobial compounds that may inhibit microorganisms by different mechanisms than those in current use [
1,
2].
Piper ovatum Vahl (Piperaceae), an herbaceous plant occurring throughout Brazil, is popularly known as “joão burandi” or “anesthetic.” It is used in traditional medicine for the treatment of inflammations and as an analgesic [
3]. The genus
Piper includes about 700 species, which are widely distributed in tropical and subtropical regions. Because they contain some substances with biological activity, several species of
Piper have been studied, and the presence of amides, lignanes, neolignanes, flavonoids, phenolic, terpenes and steroid compounds has been reported [
4,
5].
An amide isolated from
Piper species was piperine, which possesses analgesic and anti-inflammatory activity [
6]. Antimicrobial properties have also been reported for other
Piper species:
P. lanceafolium showed antifungal activity against
Candida albicans, and extracts of
P. regnellii showed antibacterial and antifungal activity [
7,
8].
Piperovatine was isolated from the leaves of
P. alatabaccum [
9]. Piperlongumine, piperovatine, isopiperlonguminine, corcovadine and isocorcovadine were obtained from
Ottonia frutens. Piperovatine is also present in other species, such as
Ottonia frutescens roots and bark,
P. piscatorum roots, and
P. longum fruits [
10,
11]. Piperovatine and piperlonguminine from
P. ovatum leaves showed excellent activity against amastigote and promastigote forms of
L. amazonensis, and the mixture of these two amides showed an anti-inflammatory effect in an ear model in mice compared with the indomethacin control [
12,
13].
Antifungal activity of essential oils extracted from
Piper aduncum, Piper arboreum and
Piper tuberculatum leaves, bark and fruits against
Cladosporium sphaerospermum and
Cladosporium cladosporioides has been reported [
14].
P. multiplinervium showed an antimicrobial effect against
Helicobacter pylori, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Mycobacterium smegmatis, Pseudomonas aeruginosa and
Candida albicans [
15]. 35 compounds have been identified by CG and CG-MS analysis of
Ambrosia trifida oil, which showed antimicrobial activity against bacteria and fungi [
16].
Candida species are harmLess saprophyte yeasts, components of the normal human biota in the gastrointestinal tract and oral and vaginal mucosae. These yeasts can cause superficial infections such as thrush and vaginitis; however, if the immune defenses of the host become compromised, they can cause severe systemic infections. Although
C. albicans is the most common fungal pathogen, infections associated with non-
albicans species have been increasing.
Candida tropicalis is the third most common species isolated [
17,
18].
Studies of plants as a source of therapeutic agents should be emphasized. In the present study, we identified active substances and essential oil compounds obtained from Piper ovatum Vahl, and investigated their antimicrobial activity in vitro.
2. Results and Discussion
Active fractions obtained from leaves of
Piper ovatum were identified as piperovatine (
1) and piperlonguminine (
2) by analyses of their
1H- and
13C-NMR data of and comparison with data from the literature [
13,
19].
Figure 1 shows the chromatogram of the crude extracts. Peak 1, with a retention time of 23.50 min, was identified as piperovatine. Peak 2, with a retention time of 24.46 min, was piperlonguminine. For component identification, the essential oil was submitted to Gas Chromatography and Mass Spectrometry (GC/MS) analysis, and the substances identified are listed in
Table 1. The components isolated in the highest quantities were
δ-amorphene (16.5 %),
cis-muurola-4(14),5-diene (14.29 %) and γ-muurolene (13.26%). The oil obtained from
P. ovatum was found to be composed of approximately equal amounts of monoterpene hydrocarbons (6.82%), oxygenated monoterpenes (2.27%), oxygenated sesquiterpene (27.27%), sesquiterpene hydrocarbons (52.27%), ketones (4.54%) and unidentified compounds (6.82%).
Figure 1.
Chromatograms of hydroalcoholic extracts of stems (A), leaves (B) and root (C) Piper ovatum; where the piperovatine (1), piperlonguminine (2). Chromatographic conditions: Metasil ODS column; mobile phase: acetonitrile:water 0 % of acetonitrile for 60 % in 30 minute and acetonitrile:water 60:40 (v/v) in isocratic for 10 minute, with 1% acetic acid; flow rate: 1.0mL/min; room temperature; detection: 280 nm.
Figure 1.
Chromatograms of hydroalcoholic extracts of stems (A), leaves (B) and root (C) Piper ovatum; where the piperovatine (1), piperlonguminine (2). Chromatographic conditions: Metasil ODS column; mobile phase: acetonitrile:water 0 % of acetonitrile for 60 % in 30 minute and acetonitrile:water 60:40 (v/v) in isocratic for 10 minute, with 1% acetic acid; flow rate: 1.0mL/min; room temperature; detection: 280 nm.
Table 1.
Main components of the essential oil from the aerial parts of P. ovatum Vahl.
Table 1.
Main components of the essential oil from the aerial parts of P. ovatum Vahl.
| Peak No. | Components | Área(%) | IK | IK* | Identification Method |
|---|
| 1 | α-Pinene | 0.27 | 930 | 939 | RT GC MS |
| 2 | β-Pinene | 0.38 | 1020 | 1024 | RT GC MS |
| 3 | Sylvestrene | 0.45 | 1024 | 1030 | RT GC MS |
| 4 | Terpinyl acetate | 0.21 | 1334 | 1349 | RT GC MS |
| 5 | Silphiperfol-4,7(14)-diene | 2.34 | 1348 | 1360 | RT GC MS |
| 6 | α-Copaene | 4.55 | 1376 | 1376 | RT GC MS |
| 7 | α-Dihydroionone | 0.14 | 1384 | 1412 | RT GC MS |
| 8 | Longifolene | 0.24 | 1390 | 1407 | RT GC MS |
| 9 | β-Elemene | 0.46 | 1392 | 1390 | RT GC MS |
| 10 | (E)-Caryophyllene | 8.49 | 1419 | 1419 | RT GC MS |
| 11 | β-Gurjunene | 1.76 | 1428 | 1433 | RT GC MS |
| 12 | (E)-
α-Ionone | 0.19 | 1433 | 1430 | RT GC MS |
| 13 | Aromadendrene | 0.22 | 1436 | 1441 | RT GC MS |
| 14 | cis-Calamenene | 1.64 | 1448 | 1529 | RT GC MS |
| 15 | α-Humulene | 0.41 | 1450 | 1460 | RT GC MS |
| 16 | Allo-Aromadendrene | 0.33 | 1459 | 1460 | RT GC MS |
| 17 | NI | 1.46 | 1470 | | |
| 18 | γ- Muurolene | 16.50 | 1476 | 1479 | RT GC MS |
| 19 | cis-Muurola-4(14),5-diene | 14.29 | 1478 | 1466 | RT GC MS |
| 20 | γ-Gurjunene | 0.39 | 1481 | 1477 | RT GC MS |
| 21 | trans-Muurola-4(14),5-diene | 1.15 | 1485 | 1493 | RT GC MS |
| 22 | δ- Cadinene | 4.89 | 1489 | 1523 | RT GC MS |
| 23 | α-Muurolene | 3.04 | 1494 | 1500 | RT GC MS |
| 24 | γ-Cadinene | 6.01 | 1508 | 1513 | RT GC MS |
| 25 | δ-Amorphene | 13.26 | 1518 | 1511 | RT GC MS |
| 26 | trans-Cadina-1,4-diene | 1.40 | 1525 | 1534 | RT GC MS |
| 27 | α-Cadinene | 0.49 | 1530 | 1538 | RT GC MS |
| 28 | α-Calacorene | 0.28 | 1535 | 1545 | RT GC MS |
| 29 | Selina-3,7(11)-diene | 0.37 | 1548 | 1546 | RT GC MS |
| 30 | trans-Dauca-4(11),7-diene | 0.26 | 1559 | 1557 | RT GC MS |
| 31 | N.I. | 0.38 | 1575 | | |
| 32 | β-Copaen-4α-ol | 0.72 | 1579 | 1590 | RT GC MS |
| 33 | Guaiol | 0.20 | 1582 | 1600 | RT GC MS |
| 34 | 1,10-di-
epi-Cubenol | 0.33 | 1593 | 1619 | RT GC MS |
| 35 | γ-Eudesmol | 0.36 | 1608 | 1632 | RT GC MS |
| 36 | cis-Cadin-4-en-7-ol | 1.73 | 1620 | 1636 | RT GC MS |
| 37 | N.I. | 0.32 | 1624 | | |
| 38 | epi- α-Muurolol | 2.77 | 1635 | 1642 | RT GC MS |
| 39 | α-Muurolol | 1.03 | 1639 | 1646 | RT GC MS |
| 40 | α-Eudesmol | 0.38 | 1644 | 1653 | RT GC MS |
| 41 | α-Cadinol | 2.03 | 1647 | 1654 | RT GC MS |
| 42 | Bulnesol | 0.24 | 1663 | 1671 | RT GC MS |
| 43 | 10-nor-Calamenen-10-one | 0.71 | 1677 | 1702 | RT GC MS |
| 44 | 7,14-anhydro-Amorpha-4,9-diene | 0.77 | 1738 | 1756 | RT GC MS |
Extracts from leaves, bark and roots of
Piper ovatum were active against
B. subtilis (250, 500 and 250 µg/mL, respectively) and
C. tropicalis ATCC 28707 (500, 250 and 62.5 µg/mL, respectively) (
Table 2). The isolated substances piperovatine and piperlonguminine showed good activity, with MIC values of 15.6 and 31.2 µg/mL, respectively, towards
B. subtilis and of 3.9 µg/mL (both) towards
C. tropicalis ATCC 28707. Piperlonguminine exhibited more activity then piperovatine against urine clinical isolates of
C. tropicalis, with MIC 31.25 µg/mL (
Table 2). Essential oil extracted from
P. ovatum leaves showed an effect against
C. tropicalis ATCC 28707 and
C. tropicalis from urine clinical isolates (22.6±3.1 and 18.7 ±2.1 mm respectively,
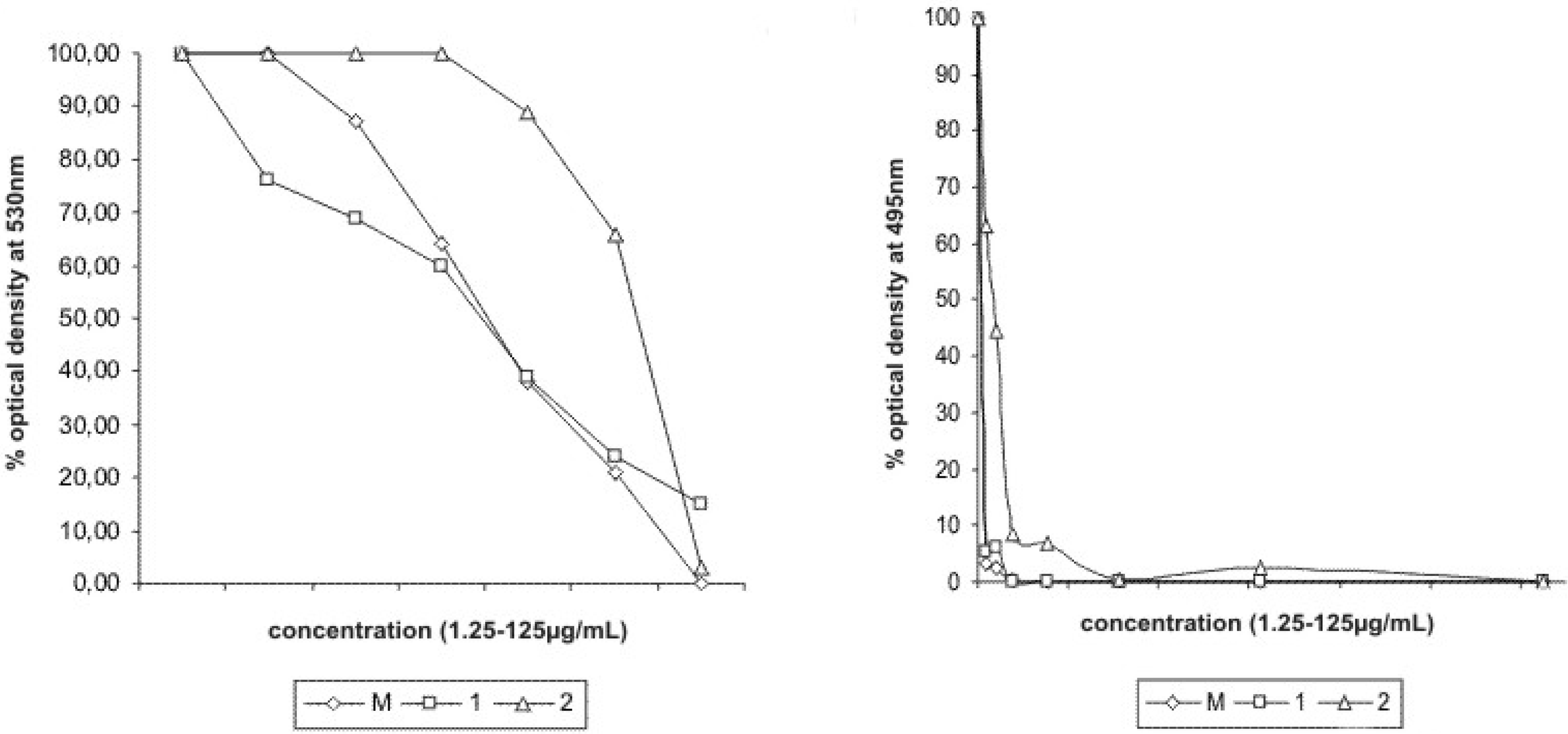
Table 3). Reduction of optical density at 530 nm and 495 nm indicated growth inhibition of
B. subtilis and
C. tropicalis at the tested concentrations from 0 to 125 µg/mL of piperovatine and piperlonguminine (
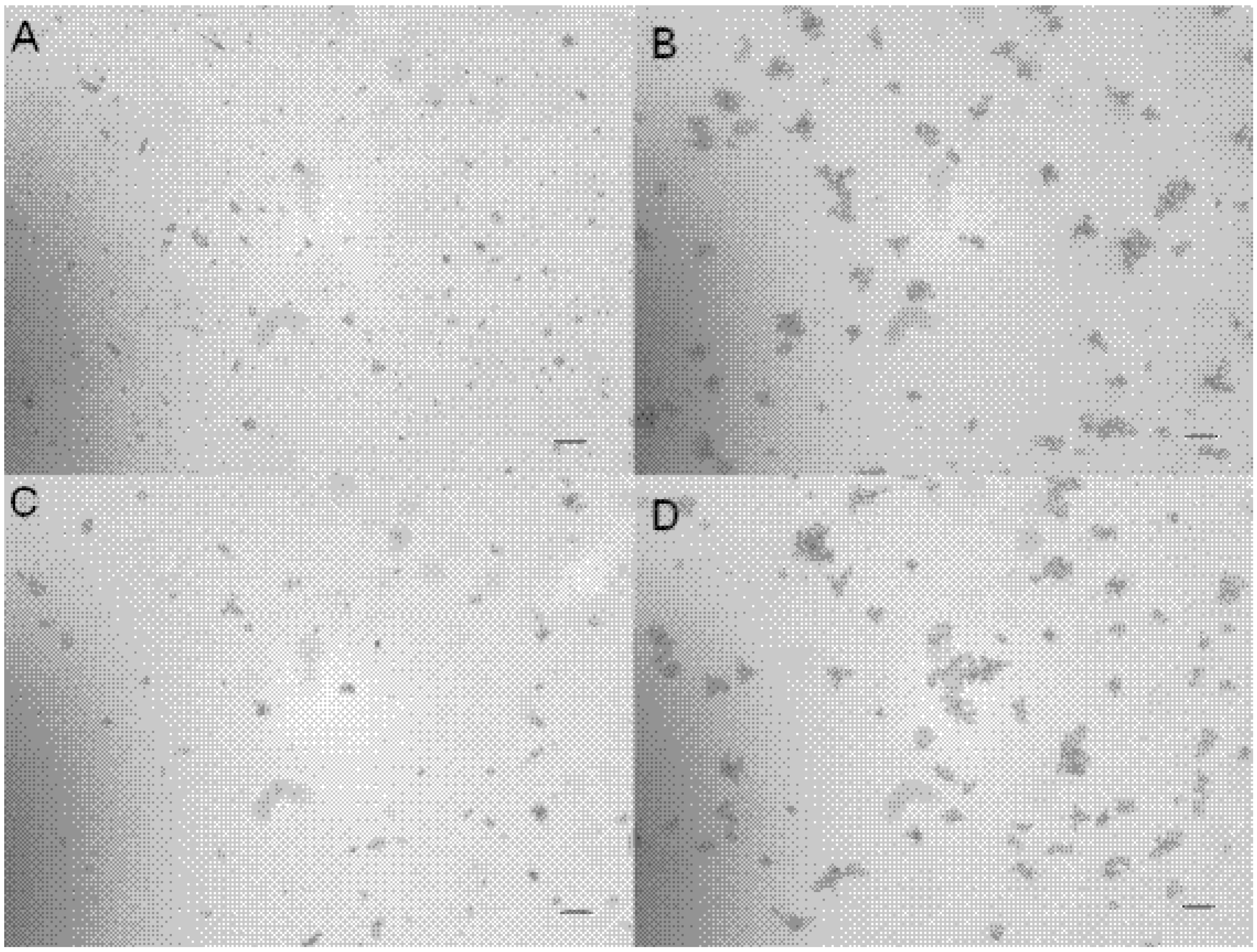
Figure 2). Both piperovatine and piperlonguminine showed an effect on the adherence of
C. tropicalis on cover glasses. When compared to untreated control yeasts, a decrease in the intensity of adhesion occurred in yeast treated with 10 µg/mL of the isolate (
Figure 3).
Table 2.
Minimal inhibitory concentration of Piper ovatum Vahl extracts and amides (µg/mL) and antibiotics used as a positive control.
Table 2.
Minimal inhibitory concentration of Piper ovatum Vahl extracts and amides (µg/mL) and antibiotics used as a positive control.
| Microrganisms | Origin | root | bark | leaves | 1 | 2 | Flu | Tetra |
|---|
| E. coli | ATCC 25922 | >1000 | >1000 | >1000 | >1000 | >1000 | | 1 |
| P. aeruginosa | ATCC 27853 | >1000 | >1000 | >1000 | >1000 | >1000 | | 12.5 |
| E. cloacae | ATCC 13047 | >1000 | >1000 | >1000 | >1000 | >1000 | | 12.8 |
| B. subtilis | ATCC 6623 | 250 | 500 | 250 | 15.6 | 31.2 | | 2.68 |
| S. aureus | ATCC 25923 | >1000 | >1000 | >1000 | >1000 | >1000 | | 0.95 |
| S. epidermidis | ATCC 12228 | >1000 | >1000 | >1000 | >1000 | >1000 | | 1.9 |
| C. tropicalis | ATCC 28707 | 62.5 | 250 | 500 | 3.9 | 3.9 | 1.9 | |
| C. albicans | ATCC 10231 | >1000 | >1000 | >1000 | >1000 | >1000 | 7.8 | |
| C. parapsilosis | ATCC 22019 | >1000 | >1000 | >1000 | >1000 | >1000 | 1.9 | |
| C. glabrata | Urine | >1000 | >1000 | >1000 | >1000 | >1000 | 31.2 | |
| C. krusei | Urine | >1000 | >1000 | >1000 | >1000 | >1000 | 62.5 | |
| C. tropicalis | catheter tip | | | | >1000 | >1000 | 3.9 | |
| C. tropicalis | Urine | | | | 250 | 250 | 3.9 | |
| C. tropicalis | orotracheal tube | | | | >1000 | >1000 | 3.9 | |
| C. tropicalis | Urine | | | | 500 | 500 | 3.9 | |
| C. tropicalis | Urine | | | | 250 | 125 | 3.9 | |
| C. tropicalis | Urine | | | | 125 | 62.5 | 3.9 | |
| C. tropicalis | Urine | | | | 125 | 31.25 | 3.9 | |
| C. tropicalis | Urine | | | | 250 | 250 | 3.9 | |
Table 3.
Antifungical activity (inhibition zone expressed in mm) investigated Piper ovatum Vahl essential oils and antibiotic used as a positive control.
Table 3.
Antifungical activity (inhibition zone expressed in mm) investigated Piper ovatum Vahl essential oils and antibiotic used as a positive control.
| Microrganisms | Origin | Pure essential oil | Nystatin |
|---|
| C. tropicalis | ATCC 28707 | 22.6±3.1 | 32 ± 2.7 |
| C. tropicalis | catheter tip | 14.3±1.1 | 27 ± 0.8 |
| C. tropicalis | Urine | 18.7 ±2.1 | 30 ± 0.3 |
| C. tropicalis | orotracheal tube | 13.8 ±0.8 | 23 ± 0.7 |
| C. tropicalis | Urine | 16.0 ±1.0 | 25 ± 1.3 |
| C. tropicalis | Urine | 11.9 ±1.1 | 35 ± 0.42 |
| C. tropicalis | Urine | 10.0±1.0 | 34 ±1.2 |
| C. tropicalis | Urine | 12.8 ±1.7 | 32 ± 2.1 |
| C. tropicalis | Urine | 14.0 ±0.7 | 26 ±0.7 |
Figure 2.
Growth inhibition of Bacillus subtilis ATCC 6623 and C. tropicalis ATCC 28707, by reduction on optical density at 530 nm and 495 nm, respectively.
Figure 2.
Growth inhibition of Bacillus subtilis ATCC 6623 and C. tropicalis ATCC 28707, by reduction on optical density at 530 nm and 495 nm, respectively.
The effect on the morphology of
C. tropicalis treated with amides extracted from
P. ovatum was investigated by scanning electron microscopy. The MIC values for piperovatine were 15.2 and 3.9 µg/mL for
B. subtilis and
C. tropicalis respectively, and the values for piperlonguminine were 31.2 and 3.9 µg/mL, indicating a selective toxicity to these microorganisms. Many studies have been conducted on antimicrobial activity, including screenings of plants in search of antimicrobial properties [
8]. Here we describe the isolation of active agents from
Piper ovatum Vahl (Piperaceae) with antimicrobial effects.
Figure 3.
Adherence inhibition assay. A and C) C. tropicalis treated with 10 µg/mL of pipeovatine and piperlonguminine, respectively. B and D) C. tropicalis without treatment (control). Bars = 50µm.
Figure 3.
Adherence inhibition assay. A and C) C. tropicalis treated with 10 µg/mL of pipeovatine and piperlonguminine, respectively. B and D) C. tropicalis without treatment (control). Bars = 50µm.
In the analysis of the essential oil of
P. aduncum, a total of 46 components were identified. The major component was identified as dill apiole or 4,5-dimethoxy-6-(2-propenyl)-1,3-benzodioxole (43.3 %), together with other minor components such as β-caryophyllene (8.3 %), piperitione (6.7 %) and α-humulene (5.1 %). Essential oil derived from
P. gibbilimbum is dominated by the gibbilimbols A-D (74.2 %), with the remaining constituents being the terpenes camphene (13.6 %) and α-pinene (6.5 %) [
20]. Data from the present study showed that the hydroalcoholic extract and the amides piperovatine and piperlonguminine from
P. ovatum Vahl have good antimicrobial activity against
B. subtilis and
C. tropicalis. Essential oil was inhibitory to
C. tropicalis. There is also an effect on adherence of
C. tropicalis on glass, and low toxic effects to cells.
4. Experimental
4.1. Plant material
Leaves of Piper ovatum Vahl were collected in December 2006 in Monte Formoso, state of Minas Gerais, Brazil, and were identified by Dr. Elsie Franklin Guimarães. A voucher specimen was deposited in the herbarium of the Department of Botany, University of Maringá (HUM 10.621).
4.2. Plant extraction and purification
Leaves were dried at room temperature and powdered (100 g). The extract was prepared by exhaustive maceration in ethanol-water (9:1 v/v) at room temperature, filtration, concentration under vacuum at 40°C to obtain a hydroalcoholic extract, and then lyophilization, which yielded 25 g of extract. The hydroalcoholic extract (14 g) was chromatographed in a vacuum silica-gel column, and eluted with gradients of hexane, dichloromethane-ethyl acetate (1:1 v/v), ethyl acetate and methanol, affording F1 (2.09 g), F2 (3.93 g), F3 (2.61 g) and F4 (3.70 g). The dichloromethane-ethyl acetate fraction F2 (3.93 g) was rechromatographed on a silica gel 60 (70-230 mesh) column chromatograph eluted with gradients of hexane, hexane/CH
2Cl
2 (98:2, 95:5, 90:10, 80:20 and 50:50 v/v), CH
2Cl
2, CH
2Cl
2/EtOAc (98:2, 95:5, 90:10, 80:20 and 50:50 v/v), EtOAc and MeOH, affording 108 fractions. Subfractions 23-38 (258.2 mg) were identified as mixtures of piperovatine and piperlonguminine. These subfractions were rechromatographed on a Sephadex LH 20, and the column chromatograph was eluted with ethyl acetate, obtaining 55 fractions. Fractions 13-30 (15 mg) and 39-48 (35 mg) were isolated and identified as piperovatine (
1) and piperlonguminine (
2) respectively, by analyses of
1H- and
13C –NMR data and comparison with data from the literature [
13,
19]. The NMR spectra were obtained on Bruker DRX-400 (8.4 T) and Varian Gemini 300 (7.05 T) spectrometers, using the deuterated solvent (CDCl
3) TMS as the internal standard and a constant temperature of 298 K. Low-resolution electrospray data were acquired in the negative ion mode, using a Micromass Quattro-LC instrument. Silica gel 60 (70-230 and 230-400 mesh); TLC: silica gel plates F
254 (0.25 mm thickness) were used for chromatographic separations.
4.3. Leaves distillation
Piper ovatum leaves (100 g) were hydrodistilled in a Clevenger-type apparatus for 3 h. The oil layers obtained were dried over anhydrous Na2SO4. The yields (2.3 % w/w) were averaged over three experiments, and calculated on the basis of the dry weight of the material. For GC studies, 1 mg of oil dissolved in 1.5 mL of hexane and 1 µL of solution was injected into the GC-MS spectrometer.
4.4. GC/MS analysis
For component identification, the essential oils were submitted to Gas Chromatography and Mass Spectrometry (GC/MS) analysis, performed using an Agilent GC (6890 Series) – quadrupole MS system (5973), equipped with a fused silica capillary column (30 m x 0.25 mm i.d. x 0.25 µm film, coated with DB-5), EI operating at 70 eV. Injector and detector temperatures were set at 250
oC. The oven temperature program was 40°C for 1 min and 40- 240 °C at 3 °C/min, and helium was employed as the carrier gas (1 mL/min). The compound was identified by comparing retention indices [Kóvats Index (KI), determined relative to the retention times of a series of
n-alkanes] [
21] and mass spectra with literature data [
22].
4.5. HPLC analysis
The HPLC analyses were carried out using a GILSON apparatus equipped with a quaternary pump (Pump 321), automatic injector valve (234) with 20 µL loop, degasifier (865), CTO-10Avp oven and a UV/visible detector model 152, controlled by a BOWTER computer program. In the chromatographic analysis, we used a reverse-phase column Metasil ODS, 5 µm, 150.0 x 4.6 mm, kept in an oven set at ambient temperature. HPLC conditions were as follows: solvent A, acetonitrile, and solvent B, 1.0 % acetic acid. A gradient elution used was 0–30 min, 0-60% A; 30–40 min, 60% A. Flow rate was 1.0 mL/min, and detection was at 280 nm. All the samples were prepared in triplicate. The reagents used to prepare the mobile phase were acetonitrile (HPLC grade from OmniSolv EM Science, Gibbstown, NJ), ultrapure water (Milli-Q system, Millipore, Bedford, USA), acetic acid (analytical grade, Merck, Darmstadt, Germany), and methanol (HPLC grade from OmniSolv EM Science, Gibbstown, NJ). The stock solutions of extracts of the leaves, stems and roots from P. ovatum were prepared in methanol at a concentration of 1,000 µg/mL. The solutions were filtered through a 0.45 µm membrane filter (Millipore, São Paulo, Brazil).
4.6. Strains and growth conditions
The test microorganisms used included Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Enterobacter cloaceae ATCC 13047, Bacillus subtilis ATCC 6623, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Candida albicans ATCC 10231, C. tropicalis ATCC 28707, C. parapsilosis ATCC 22019, and two clinical isolates of C. glabrata and C. tropicalis and one of C. krusei. Bacteria were maintained on Mueller Hinton Agar and subcultured in Mueller Hinton Broth before each experiment. Yeasts were maintained at 4 °C on Sabouraud Dextrose Agar plates and subcultured at 37 °C in Sabouraud Dextrose Broth before each experiment, to ensure viability and purity.
4.7. Microdilution MIC determination
The minimal inhibitory concentrations of the extract and oil for the strains were determined according to the M27-A2 and M7-A7 broth microdilution reference procedure of the NCCLS [
23]. RPMI 1640 medium with L-glutamine without bicarbonate buffered with 0.165 M MOPS (morpholine propanesulfonic acid) was used for yeasts, and Mueller-Hinton broth for bacteria. Serial two-fold dilutions of the extracts and isolated substances were done in a microdilution plate (96 wells) containing 100 µL of sterile medium. Next, the inoculum was added to each well. The microplates were incubated at 37 °C for 48 h for yeasts and 24 h for bacteria. The MIC was defined as the lowest concentration that resulted in inhibition of visual growth. Minimal microbicidal concentrations were determined by subculturing 10 µL of the culture from each negative well and from the positive control, measured as described.
4.8. Adherence inhibition assay
C. tropicalis (106 CFU/mL) suspension, untreated (control) and treated with decimal dilutions of piperovatine and piperlonguminine (1,000 to 0.1 µg/mL) were aliquoted (500 µL) onto a 24-well plate containing round cover glasses. The plate was incubated at 37 °C for 1 h. The cover glasses were washed with sterile phosphate buffer saline (PBS) and observed with an inverted microscope.
4.9. Scanning electron microscopy
Yeasts treated with piperovatine and piperlonguminine (3.9 µg/mL) were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2. Small drops of the fixed cells were placed on a specimen support with poly-L-lysine for 1 hour at room temperature. Subsequently, the samples were dehydrated in graded ethanol, critical-point dried in CO2, coated with gold, and examined on a Shimadzu SS-550 scanning electron microscope. Yeasts without treatment were also prepared.
4.10. Cytotoxicity assay
To investigate the cytotoxic effects of extract and isolated substances, confluent Vero and macrophage J774G8 cell monolayers grown in 96-well cell culture plates were incubated with different concentrations of extract, piperovatine and piperlonguminine for 48 h at 37 °C and 5% CO2. At the time, cultures fixed with 10% trichloroacetic acid for 1 h at 4 °C were stained for 30 min with 0.4% Sulforhodamine B (SRB) in 1% acetic acid and subsequently washed with distilled water. Bound SRB was solubilized with 150 µL 10 mM Tris-base solution. Absorbance was read in an ELISA plate reader at 530 nm. The cytotoxicity was expressed as a percentage of the optical density compared to the control.
4.11. Disc diffusion method
In vitro antifungal activity of the
P. ovatum essential oil was determined by the agar disk diffusion method according to Rubio
et al. [
24]. Briefly, a suspension of each tested microorganism (2.0 mL of 10
5 cells per mL) was carefully mixed in a tube with Mueller Hinton Agar (MHA, 18 mL), and then poured on Petri plates. Sterile filter-paper discs (Whatman No. 1, 6.0 mm in diameter) were impregnated with 15 µL of the oil and placed on the inoculated plates. Control disks containing 15 µL of the physiological saline and nystatin (100 U.I. or 20 µg/disc, Cecon, São Paulo, Brazil) were used. These plates were allowed to dry at room temperature for 2 h, and were incubated at 25ºC for 48 h. The diameters of the inhibition zones were measured in millimeters, and their means were calculated. All the tests were performed in triplicate, and the strains were tested, as listed in
Table 3.