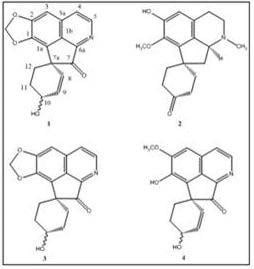
Grandine A, a New Proaporphine Alkaloid from the Bark of Phoebe grandis
Abstract
:Introduction
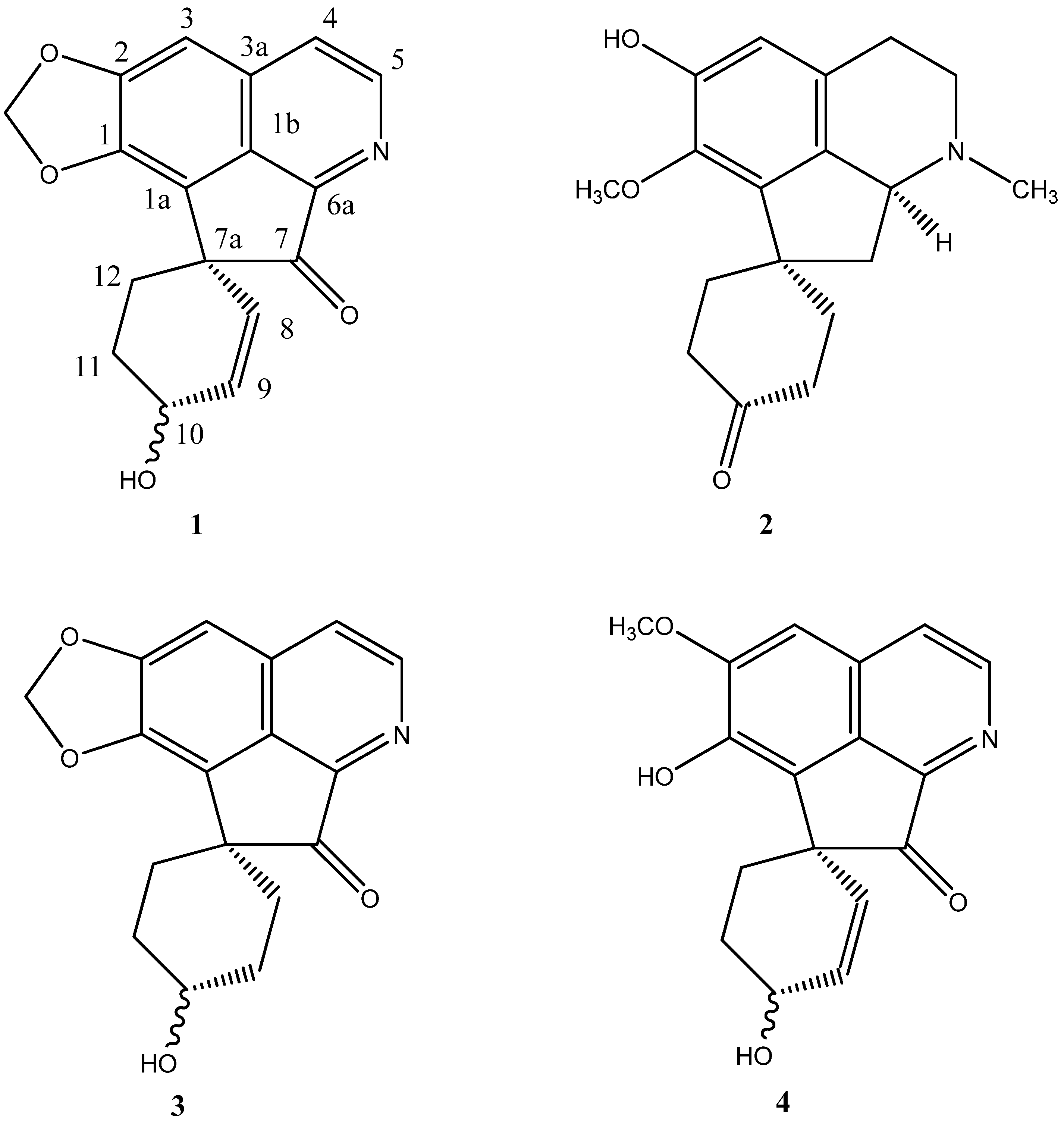
Results and Discussion
| Alkaloids | 1 | 3a | 4b | ||||
| Position | 13C | δ 1H (J Hz) | HMBC | NOESY | 13C | δ 1H (J Hz) | δ 1H (J Hz) |
| 1 | 154.59 | 154.43 | |||||
| 1a | 118.11 | 120.15 | |||||
| 1b | 136.78 | 136.39 | |||||
| 2 | 142.62 | 142.06 | |||||
| 3 | 99.76 | 7.19 s | 1, 2, 3a | OCH2O, H-4 | 99.66 | 7.04 s | 7.15 s |
| 3a | 133.11 | 133.18 | |||||
| 4 | 121.66 | 7.60 d (5.4) | 3a | H-5 | 121.58 | 7.56 d (5.4) | 7.67 d (5.6) |
| 5 | 146.92 | 8.68 d (5.4) | 3a, 4, 6a | H-4 | 146.96 | 8.66 d (5.4) | 8.77 d (5.6) |
| 6a | 150.86 | 151.23 | |||||
| 7 | 204.37 | 206.02 | |||||
| 7a | 52.73 | 50.57 | |||||
| 8 | 126.28 | 5.43 d (10.0) | 7a,10 | H-9 | 29.38 | 1.85 m (1H) 2.08 m (1H) | 5.49 dd (10, 3.2) |
| 9 | 134.75 | 6.14 dd (10.0, 2.9) | 7a, 10 | H-8, H-10 | 29.92 | 2.08 m | 6.25 dd (10, 3.2) |
| 10 | 65.05 | 4.45 br s | H-9, H-11 | 68.44 | 3.96 m | 4.44 m | |
| 11 | 28.14 | 2.38 m 2.12 m | 10, 12 | H-12 | 29.92 | 2.08 m | 2.29 m |
| 12 | 28.60 | 2.12 m (2H) | 7, 7a, 11 | H-11 | 29.38 | 1.85 m | 2.06 m 2.26 m |
| OCH2O | 102.37 | 6.16 br s 6.12 br s | 1, 2 | 102.11 | 6.13 s | ||
| 2-OMe | 4.02 s | ||||||
| Position | δ 13C | δ 1H (Hz) | DEPT | HMBC |
| 1 | 142.83 | |||
| 1a | 137.99 | |||
| 1b | 127.11 | |||
| 2 | 149.84 | |||
| 3 | 114.32 | 6.41 s | CH-3 | 1, 1b, 2, 4 |
| 3a | 131.83 | |||
| 4 | 26.72 | α 2.61 m | CH2-4 | 1b, 5 |
| β 2.55 m | ||||
| 5 | 54.76 | α 2.38 m | CH2-5 | 3a, 6a, N-CH3 |
| β 3.07 m | ||||
| 6a | 65.12 | 3.23 dd | CH-6 | 1b, 7, 7a |
| (11.0, 6.3) | ||||
| 7 | 42.21 | α 2. 72 dd (11.0, 6.3) | CH2-7 | 6a, 1b, 7a |
| β 1.65 dt (11.0) | 6a, 7a, 8, 12 | |||
| 7a | 47.29 | |||
| 8 | 33.70 | ax, 2.22 m | CH2-8 | 7 |
| eq, 1.98 m | ||||
| 9 | 38.58 | 2.48 m | CH2-9 | 8, 10 |
| 10 | 212.20 | |||
| 11 | 39.13 | ax, 2.48 m | CH2-11 | 10, 12 |
| eq, 2.80 m | ||||
| 12 | 36.23 | ax, 2.82 m | CH2-12 | 7a, 7 |
| eq, 1.82 m | ||||
| 1-OCH3 | 61.00 | 3.76 s | 1 | |
| N-CH3 | 43.06 | 2.38 s | 5, 6a |
Conclusions
Experimental
General
Plant material
Extraction and isolation of the alkaloids
Acknowledgements
References
- Mukhtar, M.R.; Martin, M.T.; Domansky, M.; Pais, M.; Hadi, A.H.A.; Awang, K. Phoebegrandines A and B, Proaporphine-Tryptamine Dimers, from Phoebe grandis. Phytochemistry 1997, 45, 1543–1546. [Google Scholar]
- Mukhtar, M.R.; Hadi, A.H.A.; Sevenet, T.; Martin, M.T.; Awang, K. Phoebegrandine C, A Novel Proaporphine-Tryptamine Dimer from Phoebe grandis (Nees) Merr. Nat. Prod. Res. 2004, 18, 163–167. [Google Scholar] [CrossRef]
- Awang, K.; Mukhtar, M.R.; Hadi, A.H.A.; Litaudon, M.; Latip, J.; Abdullah, N.R. New Alkaloids from Phoebe grandis (Nees) Merr. Nat. Prod. Res. 2006, 20, 567–572. [Google Scholar] [CrossRef]
- Awang, K.; Mukhtar, M.R.; Mustafa, M.R.; Litaudon, M.; Shaari, K.; Mohamad, K.; Hadi, A.H.A. New Alkaloids from Phoebe scortechinii. Nat. Prod. Res. 2007, 21, 704–709. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J. A. Alkaloids of Phoebe clemensii Allen (family Lauraceae). Aust. J. Chem. 1967, 20, 1277–1281. [Google Scholar] [CrossRef]
- Lu, S.T.; Tsai, I.L. Two Hexahydroproaporphine Alkaloids, Lauformine and N-Methyllauformine from Phoebe formosana. Heterocycles 1984, 22, 1031–1033. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Castro, C.O. Pentasubstituted Aporphine Alkaloids from Phoebe molicella. J. Nat. Prod. 1983, 46, 913–916. [Google Scholar] [CrossRef]
- Oscar-Castro, C.; José-López, V.; Armando-Vergara, G. Aporphine alkaloids from Phoebe pittieri. Phytochemistry 1985, 24, 203–204. [Google Scholar]
- Martínez, E.; de Díaz, A.M.P.; Nathan, P. J. Oxoaporphine Alkaloids from the Wood of Phoebe cinnamofolia. Planta Med. 1988, 361–362. [Google Scholar]
- Hegde, V.R.; Dai, P.; Ladislaw, C.; Patel, M.G.; Puar, M.S.; Pachter, J.A. ID4 Dopamine Receptor-Selective Compounds From the Chinese Plant Phoebe chekiangensis. Bioorg. Med. Chem. Lett. 1997, 7, 1207–1212. [Google Scholar]
- Mukhtar, M.R.; Hadi, A.H.A; Rondeau, D.; Richomme, P.; Litaudon, M.; Mustafa, M.R.; Awang, K. New proaporphines from the bark of Phoebe scortechinii. Nat. Prod. Res. 2008, 22, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Haynes, L.J.; Stuart, K.L.; Barton, D.H.R.; Kirby, G.W. Alkaloids from Croton species. Part III. The Constitution of the Proaporphines Crotonosine, “Homolinearine”, Base A, and the Dihydro-proaporphine linearisine. J. Chem. Soc. C 1966, 19, 1676–1679. [Google Scholar]
- Haynes, L.J.; Husbands, G.E.M.; Stuart, K.L. Alkaloids from Croton species. IV. The isolation and structure of a new proaporphine. J. Chem. Soc. C 1966, 19, 1680–1681. [Google Scholar]
- Snatzke, G.; Wollenberg, G. Alkaloids from Croton species. V. Configuration of linearisine and circular dichroism of proaporphine-type alkaloids. J. Chem. Soc. C 1966, 19, 1681–1685. [Google Scholar] [CrossRef]
- Kametani, T.; Yagi, H. Total syntheses of (±)-Glaziovine and (±)-Pronuciferine by Phenolic Oxidative coupling. J. Chem. Soc. C 1967, 20, 2182–2184. [Google Scholar] [CrossRef]
- Gözler, B.; Guinaudeau, H.; Shamma, M.; Sariyar, G. The photolysis of proaporphines Tetrahedron Lett. 1986, 27, 1899–1902. [Google Scholar]
- Wu, T.-S.; Lin, F.-W. Alkaloids of the Wood of Cryptocarya chinensis. J. Nat. Prod. 2001, 64, 1404–1407. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mukhtar, M.R.; Aziz, A.N.; Thomas, N.F.; Hadi, A.H.A.; Litaudon, M.; Awang, K. Grandine A, a New Proaporphine Alkaloid from the Bark of Phoebe grandis. Molecules 2009, 14, 1227-1233. https://doi.org/10.3390/molecules14031227
Mukhtar MR, Aziz AN, Thomas NF, Hadi AHA, Litaudon M, Awang K. Grandine A, a New Proaporphine Alkaloid from the Bark of Phoebe grandis. Molecules. 2009; 14(3):1227-1233. https://doi.org/10.3390/molecules14031227
Chicago/Turabian StyleMukhtar, Mat Ropi, Ahmad Nazif Aziz, Noel F. Thomas, A. Hamid A. Hadi, Marc Litaudon, and Khalijah Awang. 2009. "Grandine A, a New Proaporphine Alkaloid from the Bark of Phoebe grandis" Molecules 14, no. 3: 1227-1233. https://doi.org/10.3390/molecules14031227