Polyphenolic Profile and Bioactivity Study of Oenothera speciosa Nutt. Aerial Parts
Abstract
:1. Introduction
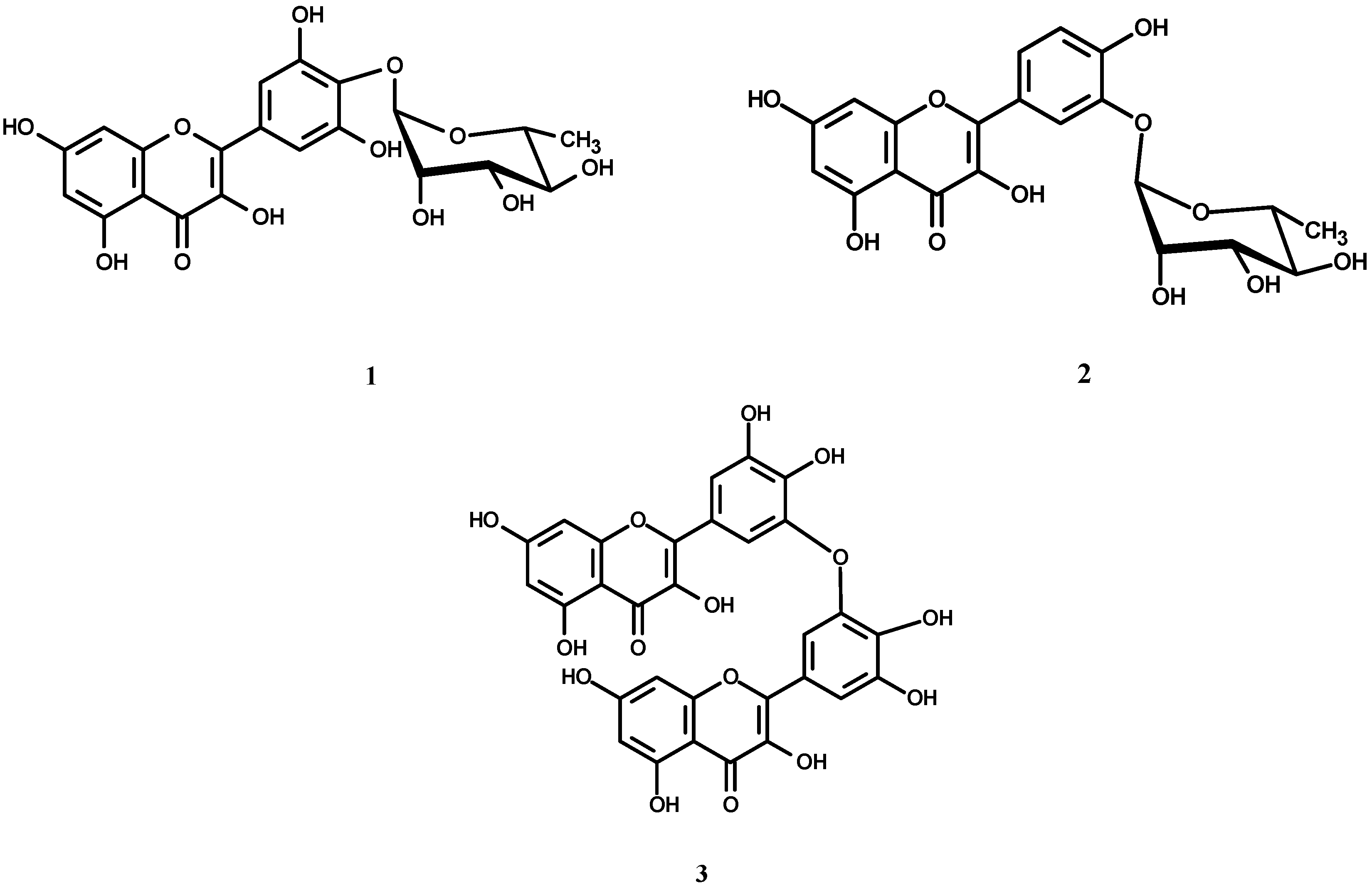
2. Results and Discussion
| Groups | Oedema | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 hour | 2 hours | 3 hours | 4 hours | |||||
| % Increase | Potency | % Increase | Potency | % Increase | Potency | % Increase | Potency | |
| Control | 96.96 ± 5.6 | ----- | 107.81 ± 8.7 | ----- | 110.03 ± 7.7 | ----- | 110.76 ± 7.9 | ----- |
| Group/dosemg/kg b.wt. | Glucose mg/dL in diabetic rats | ||
|---|---|---|---|
| Before treatment | After treatment | ||
| Zero | 15 days | 30 days | |
| X± S.E | X± S.E | X± S.E | |
| Normal control | 78.3 ± 3.2 | 75.4 ± 5.3 | 72.2 ± 2.8 |

3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.4. Animals
3.5. Determination of median lethal dose (LD50)
3.6. Determination of anti-inflammatory activity
3.7. Determination of antihyperglycaemic activity
3.8. Determination of antioxidant activity

4. Conclusions
Acknowledgements
References and Notes
- Bailey, L.H. Manual of cultivated plants. Revised Edition, Revised Edition; The Macmillan Company: New York, USA, 1958; pp. 733–739. [Google Scholar]
- Lawrence, G.H.M. Taxonomy of vascular plants; The Macmillan Company: New York, USA, 1958; pp. 637–639. [Google Scholar]
- Hudson, B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc. 1984, 61, 540–543. [Google Scholar] [CrossRef]
- Michimasa, H. Antioxidant and antiaging effect of Evening Primrose seed polyphenols. Fragr. J. 2004, 32, 82–87. [Google Scholar]
- Yoshida, T.; Hatano, T.; Chou, T.; Yasuhara, T.; Matsuda, M.; Yazaki, K.; Okuda, T.; Miyamoto, K.; Koshiura, R.; Nitta, A. Antitumor hydrolysable tannin oligomers with macrocyclic structures. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 1990, 32, 221–228. [Google Scholar]
- Taniguchi, S.; Imayoshi, Y.; Yabu-uchi, R.; Ito, H.; Hatano, T.; Yoshida, T. A macrocyclic ellagitannin trimer oenotherin T1 from Oenothera species. Phytochemistry 2002, 59, 191–195. [Google Scholar] [CrossRef]
- Abdullazhanova, N.G.; Mavlyanov, S.M.; Abdullaev, S.V. Phenolic compounds from Oenothera gigas. Chem. Nat. Compd. 2000, 36, 97–98. [Google Scholar] [CrossRef]
- Yoshida, T.; Chou, T.; Shingu, T.; Okuda, T. Oenotheins D, F, and G, hydrolysable tannin dimers from Oenothera lacinia. Phytochemistry 1995, 40, 555–561. [Google Scholar] [CrossRef]
- Hatano, T.; Yasuhara, T.; Matsuda, M.; Yazaki, K.; Yoshida, T.; Okuda, T. Oenothein B, a dimeric, hydrolysable tannin with macrocyclic structure, and accompanying tannins from Oenothera erythrosepala. J. Chem. Soc., Perkin Trans. 1 1990, 10, 2735–2743. [Google Scholar]
- Zadernowski, R.; Naczk, M.; Nowak-Polakowska, H. Phenolic acids of borage (Borage officinalis L.) and evening primrose (Oenothera biennis L.). J. Am. Oil Chem. Soc. 2002, 79, 335–338. [Google Scholar] [CrossRef]
- Krzaczek, T.; Bogucka, K.A.; Sniezko, R. The phenolic acids of some species of the Oenothera L. genus. Acta Soc. Bot. Pol. 1995, 64, 41–44. [Google Scholar] [CrossRef]
- Shukla, Y.N.; Srivastava, A.; Kumar, S.; and Kumar, S. Phytotoxic and antimicrobial constituents of Argyreia speciosa and Oenothera biennis. J. Ethnopharmacol. 1999, 67, 241–245. [Google Scholar] [CrossRef]
- Nakanishi, T.; Inatomi, Y.; Murata, H.; Ishida, S.S.; Fujino, Y.; Miura, K.; Yasuno, Y.; Inada, A.; Lang, F.A.; Murata, J. Triterpenes and flavonol glucuronides from Oenothera cheiranthifolia. Chem. Pharm. Bull. 2007, 55, 334–336. [Google Scholar] [CrossRef]
- Zinsmeister, H.D.; Plitzko, I.; Schels, H. Flavonol glycosides in South American species of Oenothera section Oenothera. Phytochemistry 1977, 16, 497. [Google Scholar] [CrossRef]
- Zinsmeister, H.D.; Biering, W. Onagraceae, flavonol-glycosides in Oenothera hookeri. Phytochemistry 1973, 12, 234. [Google Scholar] [CrossRef]
- Neumann, G.; Schwemmle, B. Flavonoids from Oenothera-seedlings: identification and extra nuclear control of their biosynthesis. J. Plant Physiol. 1993, 142, 135–143. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The ultraviolet spectra of flavones and flavonols. In The systematic identification of flavonoids; Springer Verlag: New York, USA, 1970; pp. 41–164. [Google Scholar]
- Agrawal, P.K. Flavonoid Glycosides. In Studies in Organic Chemistry 39, Carbon-13 NMR of Flavonoids; Agrawal, P.K., Bansal, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 283–364. [Google Scholar]
- Harborne, J.B. Flavone and flavone glycosides. In The flavonoids: Advances in Research since 1986; Williams, C.A., Harborne, J.B., Eds.; Chapman & Hall: Cambridge, UK, 1994; pp. 337–385. [Google Scholar]
- Harbone, J.B. Phytochemical methods: A Guide to Modern Techniques of Plant Analysis., 2nd Edition ed; Chapman & Hall: London, UK, 1984; pp. 49–50. [Google Scholar]
- Markham, K.R.; Chari, V.M. Carbon-13 NMR spectroscopy of flavonoids. In The Flavonoids: Advances in Research; Harborne, J.B., Mabry, T.J., Eds.; Chapman and Hall: London, UK, 1982; pp. 19–132. [Google Scholar]
- Semler, D.E. The rat. In Animal models in toxicology; Gad, S.C., Chengelis, C.P., Eds.; Marcel Dekker: New York, USA, 1992; p. 39. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenan-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Obukowicz, M.G.; Welsch, D.J.; Salsgiver, W.J.; Martin-Berger, C.L.; Chinn, K.S.; Duffin, K.L.; Raz, A.; Needlemann, P. Novel, selective delta 6 or delta 5 fatty acid desaturase inhibitors as anti-inflammatory agents in mice. J. Pharmacol. Exp. Ther. 1998, 287, 157–166. [Google Scholar]
- Rakieten, N.; Rakieten, M.L.; Nadkarni, M.V. Studies on the diabetic action of streptozotocin. Cancer Chemother. Rep. 1963, 29, 91–98. [Google Scholar]
- Bedir, A.; Aliyazicioglu, Y.; Kahraman, H.; Yurdakul, Z.; Uysal, M.; Suvaci, D.E.; Okuyucu, A.; Hokelek, M.; Alvur, M. Genotoxicity in rats treated with antidiabietic agent, rosiglitazone. Environ. Mol. Mutagen. 2006, 47, 718–724. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Govindarajan, R.; Rastogi, S.; Vijayakumar, M.; Rawat, A.K.S.; Shirwaikar, A.; Mehrotra, S.; Pushpangadan, P. Studies on the antioxidant activities of Desmodium gangeticum. Biol. Pharm. Bull. 2003, 26, 1424–1427. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marzouk, M.S.; Moharram, F.A.; El Dib, R.A.; El-Shenawy, S.M.; Tawfike, A.F. Polyphenolic Profile and Bioactivity Study of Oenothera speciosa Nutt. Aerial Parts. Molecules 2009, 14, 1456-1467. https://doi.org/10.3390/molecules14041456
Marzouk MS, Moharram FA, El Dib RA, El-Shenawy SM, Tawfike AF. Polyphenolic Profile and Bioactivity Study of Oenothera speciosa Nutt. Aerial Parts. Molecules. 2009; 14(4):1456-1467. https://doi.org/10.3390/molecules14041456
Chicago/Turabian StyleMarzouk, Mohamed S., Fatma A. Moharram, Rabab A. El Dib, Siham M. El-Shenawy, and Ahmed F. Tawfike. 2009. "Polyphenolic Profile and Bioactivity Study of Oenothera speciosa Nutt. Aerial Parts" Molecules 14, no. 4: 1456-1467. https://doi.org/10.3390/molecules14041456




