New Synthesis and Antiparasitic Activity of Model 5-Aryl-1-methyl-4-nitroimidazoles
Abstract
:Introduction

Results and Discussion
Chemistry
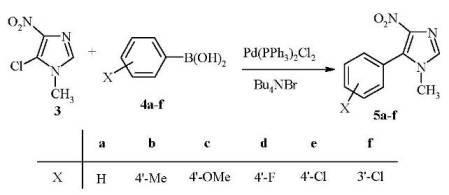
 6B) [23]. This step led to a mixture of 5a and its isomeric 1-methyl-4-aryl-5-nitroimidazole 7A, the isolation of which required extra separation and purification efforts. Additionally, the required synthon (6A
6B) [23]. This step led to a mixture of 5a and its isomeric 1-methyl-4-aryl-5-nitroimidazole 7A, the isolation of which required extra separation and purification efforts. Additionally, the required synthon (6A  6B) was prepared by condensation of (substituted)phenacyl chloride with formamide, followed by acidification and then neutralization with aqueous ammonium hydroxide [24].
6B) was prepared by condensation of (substituted)phenacyl chloride with formamide, followed by acidification and then neutralization with aqueous ammonium hydroxide [24]. 

Antiamoebic and antigiardial activity
| Mean IC50 ± SD(n) (μM) | ||||
|---|---|---|---|---|
| Compound | Giardia intestinalis | Entamoeba histolytica | Hep-2 cells | Vero cells |
| 5a | 4.43 ± 1.97 | 4.04 ± 0.28 | 1040.27 ± 19.18 | 1748.28 ± 18.38 |
| 5b | 4.01 ± 0.75 | 3.10 ± 0.41 | 1610.74 ± 22.23 | 1633.32 ± 13.61 |
| 5c | 1.72 ± 0.57 | 1.16 ± 0.19 | 568.80 ± 22.71 | 868.24 ± 22.02 |
| 5d | 3.76 ± 0.2 | 4.39 ± 0.71 | 1894.12 ± 21.13 | 1918.41 ± 13.37 |
| 5e | 1.90 ± 0.14 | 1.56 ± 0.156 | 437.19 ± 16.39 | 725.05 ± 11.79 |
| 5f | 1.47 ± 0.14 | 1.89 ± 0.14 | 1780.21 ± 15.71 | 1783.16 ± 19.66 |
| Metronidazole | 4.39 ± 0.59 | 4.10 ± 0.78 | 2044.20 ± 26.36 | 2071.35 ± 16.37 |
| IC50 Ratio | ||||
|---|---|---|---|---|
| (metronidazole/compound) | ||||
| Compound | Giardia intestinalis | Entamoeba histolytica | Hep-2 cells | Vero cells |
| 5a | 1 | 1 | 2 | 1.2 |
| 5b | 1.1 | 1.3 | 1.3 | 1.3 |
| 5c | 3.5 | 2.6 | 3.6 | 2.4 |
| 5d | 1.2 | 0.93 | 1.1 | 1.1 |
| 5e | 2.6 | 2.3 | 4.7 | 2.9 |
| 5f | 2.2 | 2.9 | 1.2 | 1.2 |
Experimental
General
General procedure for the synthesis of 5-aryl-1-methyl-4-nitroimidazoles 5a-f
Biological Activity
Test organisms
Antiamoebic and antigiardial activity
Cytotoxicity assay
Conclusions
Acknowledgements
- Samples Availability: Samples of compounds 5a-f are available from the authors.
References and Notes
- Boiani, M.; Gonzalez, M. Imidazole and benzimidazole derivatives as chemotherapeutic agents. Mini-Rev. Med. Chem. 2005, 5, 409–424. [Google Scholar] [CrossRef]
- De Luca, L. Naturally occurring and synthetic imidazoles: Their chemistry and their biological activities. Curr. Med. Chem. 2006, 13, 1–23. [Google Scholar]
- Du, H.; He, Y.; Rasapalli, S.; Lovely, C.J. Newmethods of imidazole functionalization from imidazole to marine alkaloids. Synlett 2006, 7, 965–992. [Google Scholar]
- Grimmett, M.R. Imidazoles and their benzo derivatives. In Comprehesive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Potts, K.T., Eds.; Pergamon Press: Oxford, UK, 1984; Vol. 5 (Part 4A), pp. 345–497. [Google Scholar]
- Ganellin, C.R. Discovery of the antiulcer drug Tagamet. Drug Discov. Dev. 2006, 1, 295–311. [Google Scholar] [CrossRef]
- Silverman, R.A. The Organic Chemistry of Drug Design and Drug Action; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; p. 159. [Google Scholar]
- Yokoyama, M.; Aono, H.; Takeda, A.; Morita, K. Cimetidine for chronic calcifying tendinitis of the shoulder. Reg. Anesth. Pain Med. 2003, 28, 248–252. [Google Scholar]
- Matsuo, Y. Pharmacology of cimetidine. Kansen Ensho Men'eki 1983, 13, 217–228. [Google Scholar]
- Muller, C.E. Basic chemistry of 2-nitroimidazoles (azomycin derivatives). Dev. Nucl. Med. 1999, 33, 47–59. [Google Scholar]
- Hori, H.; Jin, C.Z.; Kiyono, M.; Kasai, S.; Shimamura, M.; Inayama, S. Design, synthesis, and biological activity of anti-angiogenic hypoxic cell radiosensitizer haloacetylcarbamoyl-2-nitroimidazoles. Bioorg. Med. Chem. 1997, 5, 591–599. [Google Scholar] [CrossRef]
- Lawton, C.A.; Coleman, C.N.; Buzydlowski, J.W.; Forman, J.D.; Marcial, V.A.; DelRowe, J.D.; Rotman, M. Results of a phase II trial of external beam radiation with etanidazole (SR 2508) for the treatment of locally advanced prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 673–680. [Google Scholar] [CrossRef]
- Maurin, M.B.; Rowe, S.M.; Field, K.S.; Swintosky, R.C.; Hussain, M.A. Solubility behavior, phase transition, and structure-based nucleation inhibition of etanidazole in aqueous solutions. Pharm. Res. 1996, 13, 1401–1405. [Google Scholar] [CrossRef]
- Bendesky, A.; Menendez, D. Metronidazole: A comprehensive review. Rev. Fac. Med. U.N.A.M. 2001, 44, 255–259. [Google Scholar]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole: A therapeutic review and update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Goldman, P.; Wuest, J.D. Reactions of nitroimidazoles. Nucleophilic substitution of the nitro group. J. Am. Chem. Soc. 1981, 103, 6224–6226. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Metronidazole in anaerobic infections: A review of its activity, pharmacokinetics and therapeutic use. Drugs 1978, 16, 387–417. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kumar, S.; Seth, M.; Bhaduri, A.P. Synthesis of 1-methyl-4-nitro-5- substituted imidazole and substituted imidazolothiazole derivatives as possible antiparasitic agents. Indian J. Chem. Section B: Org. Chem. Incl. Med. Chem. 1989, 28B, 391–396. [Google Scholar]
- Thomas, A.H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J. Antimicrob. Chemother. 1986, 17, 269–279. [Google Scholar] [CrossRef]
- Egolf, R.A.; Heindel, N.D. The synthesis of aryl 4-nitro-5-imidazolyl sulfone radiation sensitizers sterically protected against glutathione reaction. J. Heterocycl. Chem. 1991, 28, 577–582. [Google Scholar] [CrossRef]
- Shafiee, A.; Shahocini, S. Nitroimidazoles. V. Synthesis of 1-methyl-2-(2-methyl-4-thiazolyl)-nitroimidazoles. J. Heterocycl. Chem. 1989, 26, 1627–1629. [Google Scholar]
- Boechat, N.; Carvalho, A.S.; Fernandes-Ferreira, E.; Soares, R.O.A.; Souza, A.S.; Gibaldi, D.; Bozza, M.; Pinto, A.C. Novel nitroimidazoles with trypanocidal and cell growth inhibition activities. Cytobios 2001, 105, 83–90. [Google Scholar]
- Carvalho, A.S.; Gibaldi, D.; Pinto, A.C.; Bozza, M.; Boechat, N. Synthesis and trypanocidal evaluation of news 5-[N-(3-(5-substituted)-1,3,4-thiadiazolyl)]amino-1- methyl-4-nitroimidazoles. Lett. Drug Design Discov. 2006, 3, 98–101. [Google Scholar] [CrossRef]
- Ehlhardt, W.J.; Beaulieu, B.B.; Goldman, P. Nitrosoimidazoles: Highly bactericidal analogs of 5-nitroimidazole drugs. J. Med. Chem. 1988, 31, 323-329, and references cited therein. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Lord, G.H. Substituted imidazoles. Brit. Pat. GB 1 046 248, 1966. [Chem. Abstr. 1967, 66, 37927]. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Stanforth, S.P. Catalytic cross-coupling reactions in biaryl synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar] [CrossRef]
- Chemeler, S.R.; Trauner, D.; Danishefsky, S.J. The B-alkyl Suzuki-Miyaura cross- coupling reaction: Development, mechanistic study, and applications in natural product synthesis. Angew. Chem. Int. Ed. Engl. 2001, 40, 4544–4568. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Zapf, A. Coupling of aryl and alkyl halides with organoboron reagents (Suzuki Reaction). In Transition Metals for Organic Synthesis, 2nd; Beller, M., Bolm, C., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 211–229. [Google Scholar]
- Shortly after completion of the present work, a synthesis of 4,5-diaryl-1-methylimidazoles by Pd-catalyzed direct coupling reaction has been reported: Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Rossi, R. Regioselective synthesis of 4,5-diaryl-1-methyl-1H-imidazoles including highly cytotoxic derivatives by Pd-catalyzed direct C-5 arylation of 1-methyl-1H-imidazole with aryl bromides. Eur. J. Org. Chem. 2008, 32, 5436–5445.
- Reetz, M.T.; Westermann, E. Phosphane-free palladium-catalyzed coupling reactions: The decisive role of Pd nanoparticles. Angew. Chem. Int. Ed. Engl. 2000, 39, 165–168. [Google Scholar] [CrossRef]
- Knight, R. The chemotherapy of amebiasis. J.Antimicrob. Chemother. 1980, 6, 577–593. [Google Scholar] [CrossRef]
- Majewska, A.C.; Kasprzak, W.; De Jonckheere, J.F.; Kaczmarek, E. Heterogeneity in the sensitivity of stocks and clones of Giardia to metronidazole and ornidazole. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 67–69. [Google Scholar] [CrossRef]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Mosleh, I.M.; Mubarak, M.S. Synthesis of novel hybrid molecules from precursors with known antiparasitic activity. Molecules 2009, 14, 1483–1494. [Google Scholar] [CrossRef]
- Aley, S.B.; Zimmerman, M.; Hetsko, M.; Selsted, M.E.; Gillin, F.D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 1994, 62, 5397–5403. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saadeh, H.A.; Mosleh, I.M.; El-Abadelah, M.M. New Synthesis and Antiparasitic Activity of Model 5-Aryl-1-methyl-4-nitroimidazoles. Molecules 2009, 14, 2758-2767. https://doi.org/10.3390/molecules14082758
Saadeh HA, Mosleh IM, El-Abadelah MM. New Synthesis and Antiparasitic Activity of Model 5-Aryl-1-methyl-4-nitroimidazoles. Molecules. 2009; 14(8):2758-2767. https://doi.org/10.3390/molecules14082758
Chicago/Turabian StyleSaadeh, Haythem A., Ibrahim M. Mosleh, and Mustafa M. El-Abadelah. 2009. "New Synthesis and Antiparasitic Activity of Model 5-Aryl-1-methyl-4-nitroimidazoles" Molecules 14, no. 8: 2758-2767. https://doi.org/10.3390/molecules14082758