Evolution of the Yields and Composition of Essential Oil from Portuguese Myrtle (Myrtus comunis L.) through the Vegetative Cycle
Abstract
:1. Introduction
2. Results and Discussion
| Month | Stage | Yield(g/100 g dried leaves) | ||
|---|---|---|---|---|
| Leaves | Branches | Berries | ||
| May | Pre-flower | 0.33±0.03 | 0.02±0.01 | - |
| June | Flower buds | 0.60±0.04 | 0.19±0.01 | - |
| July | Flower blossom | 0.48±0.01 | 0.17±0.03 | - |
| September | Unripe fruit | 0.56±0.05 | 0.18±0.01 | 0.23±0.04 |
| October | Ripe fruit | 0.74±0.09 | 0.07±0.01 | 0.11±0.06 |
| Components a | LRI b | ID Method c | Composition (w/w%) ± SD e | ||||
|---|---|---|---|---|---|---|---|
| May | June | July | September | October | |||
| Tricyclened | 908 | MS, LRI | 0.08±0.00 | 0.12±0.00 | 0.08±0.00 | 0.14±0.00 | 0.19±0.00 |
| α -Thujened | 917 | MS, LRI | 0.05±0.00 | 0.08±0.00 | 0.10±0.00 | 0.06±0.00 | 0.05±0.00 |
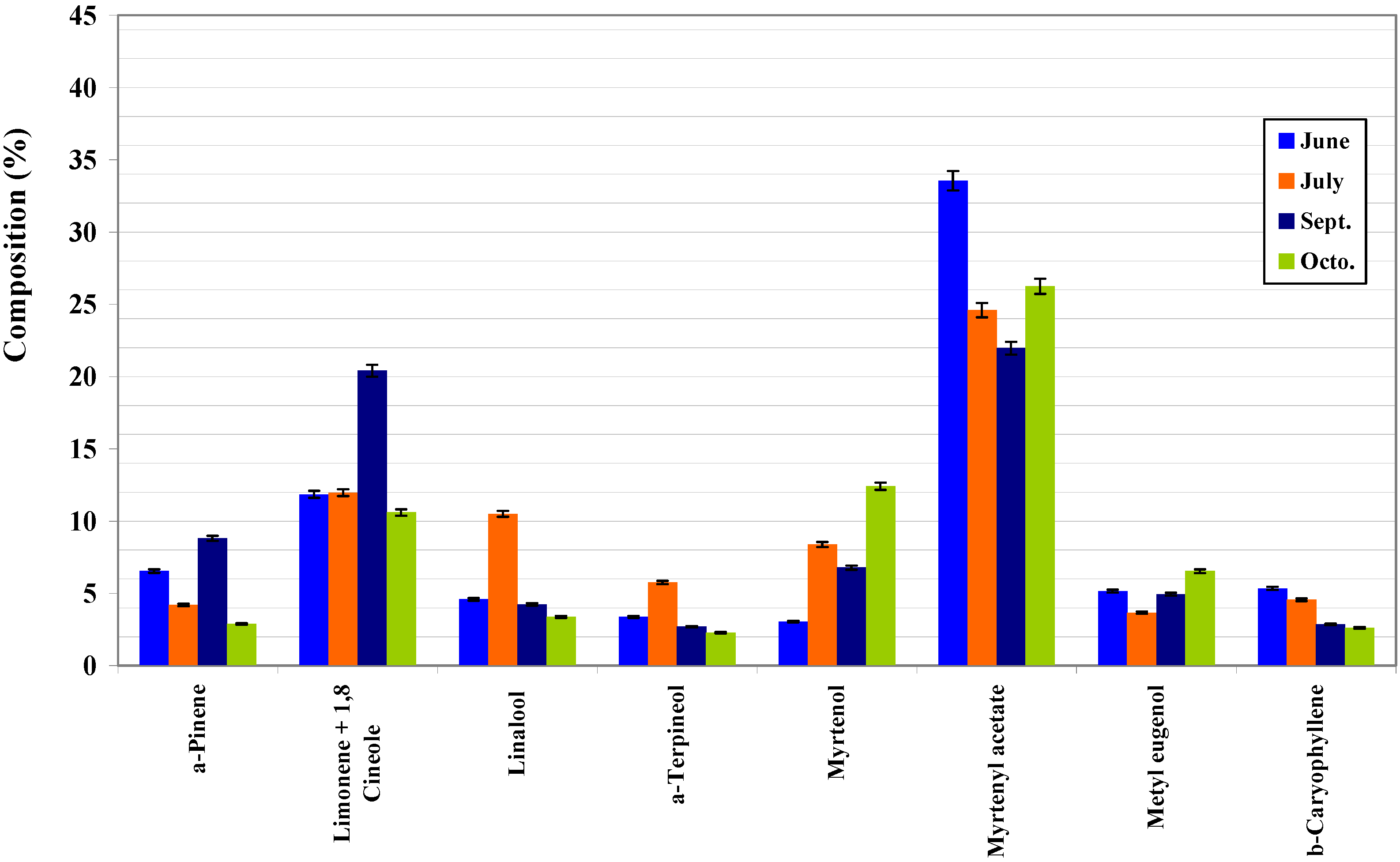
| α-Pinene | 922 | MS, LRI, std | 10.38±0.07 | 16.45±0.01 | 21.50±0.10 | 13.12±0.22 | 15.25±0.01 |
| Camphened | 934 | MS, LRI | 0.03±0.00 | 0.03±0.00 | 0.04±0.00 | 0.03±0.00 | 0.03±0.01 |
| β-Pinene | 963 | MS, LRI, std | 0.12±0.00 | 0.16±0.00 | 0.26±0.00 | 0.16±0.01 | 0.19±0.00 |
| Myrcene | 985 | MS, LRI, std | 0.11±0.00 | 0.09±0.00 | 0.14±0.00 | 0.08±0.01 | 0.08±0.00 |
| δ-3-Carened | 998 | MS, LRI | 0.02±0.00 | 0.12±0.00 | 0.05±0.00 | 0.18±0.00 | 0.02±0.00 |
| α-Terpinene | 1001 | MS, LRI, std | 0.17±0.01 | 0.12±0.00 | 0.11±0.01 | 0.13±0.00 | 0.19±0.01 |
| p-Cymene | 1014 | MS, LRI, std | 0.04±0.00 | 0.03±0.00 | 0.03±0.00 | 0.04±0.00 | 0.04±0.00 |
| Limonene+1,8-Cineole | 1024 | MS, LRI, std | 20.03±0.11 | 31.33±0.13 | 39.45±0.10 | 32.13±0.22 | 36.78±0.09 |
| o-Cymened | 1037 | MS, LRI | 0.01±0.00 | 0.01±0.00 | 0.02±0.00 | 0.06±0.00 | 0.06±0.00 |
| γ-Terpinene | 1045 | MS, LRI, std | 0.21±0.00 | 0.11±0.00 | 0.11±0.00 | 0.18±0.00 | 0.15±0.00 |
| Linalool oxide | 1059 | MS, LRI, std | 0.04±0.00 | 0.20±0.00 | 0.30±0.00 | 0.05±0.00 | 0.05±0.00 |
| α-Terpinolened | 1080 | MS, LRI | 0.10±0.00 | 0.20±0.00 | 0.25±0.01 | 0.09±0.00 | 0.07±0.00 |
| Linalool | 1096 | MS, LRI, std | 7.58±0.06 | 7.01±0.02 | 6.19±0.03 | 9.27±0.03 | 7.91±0.01 |
| Fenchol | 1100 | MS, LRI, std | 1.10±0.01 | 0.57±0.01 | 0.52±0.02 | 0.75±0.01 | 0.73±0.03 |
| Trans-inocarveold | 1129 | MS, LRI | 0.15±0.00 | 0.04±0.00 | 0.04±0.00 | 0.10±0.00 | 0.15±0.00 |
| Borneol | 1160 | MS, LRI, std | 0.06±0.00 | 0.08±0.00 | 0.14±0.00 | 0.08±0.00 | 0.08±0.01 |
| Terpinen-4-ol | 1169 | MS, LRI, std | 0.19±0.00 | 0.22±0.00 | 0.29±0.00 | 0.25±0.00 | 0.25±0.00 |
| α-Terpineol | 1183 | MS, LRI, std | 2.69±0.03 | 3.63±0.01 | 5.15±0.02 | 3.34±0.01 | 3.46±0.00 |
| Myrtenol | 1188 | MS, LRI, std | 2.86±0.07 | 0.79±0.00 | 0.32±0.00 | 1.94±0.01 | 1.85±0.00 |
| Geraniol | 1252 | MS, LRI, std | 0.89±0.00 | 0.84±0.01 | 0.89±0.00 | 0.80±0.01 | 0.94±0.00 |
| Borneol acetate | 1278 | MS, LRI, std | 0.02±0.01 | 0.012±0.00 | 0.02±0.00 | 0.01±0.00 | 0.02±0.00 |
| Trans pinocarvyl acetated | 1292 | MS, LRI | 0.64±0.00 | 0.39±0.01 | 0.16±0.01 | 0.46±0.05 | 0.39±0.00 |
| Myrtenyl acetate | 1323 | MS, LRI, std | 37.62±0.13 | 22.19±0.09 | 7.40±0.02 | 24.83±0.21 | 20.75±0.02 |
| α-Terpenyl acetated | 1336 | MS, LRI | 0.33±0.00 | 0.30±0.00 | 0.22±0.00 | 0.19±0.00 | 0.23±0.00 |
| Eugenold | 1343 | MS, LRI | 0.33±0.01 | 0.84±0.00 | 1.54±0.01 | 0.49±0.00 | 0.09±0.00 |
| Neryl acetate | 1363 | MS, LRI, std | 0.06±0.00 | 0.09±0.00 | 0.12±0.02 | 0.05±0.00 | 0.04±0.00 |
| Geranyl acetate | 1376 | MS, LRI, std | 0.38±0.00 | 0.24±0.00 | 0.11±0.00 | 0.21±0.00 | 0.14±0.00 |
| Methyl eugenold | 1381 | MS, LRI | 2.13±0.01 | 3.52±0.01 | 4.26±0.02 | 2.18±0.03 | 1.71±0.00 |
| β-Caryophyllene | 1402 | MS, LRI, std | 2.57±0.01 | 2.87±0.01 | 2.18±0.01 | 1.73±0.01 | 1.83±0.00 |
| α-Humulene | 1442 | MS, LRI, std | 0.88±0.00 | 0.51±0.01 | 0.84±0.01 | 0.26±0.01 | 0.17±0.00 |
| Geranyl isobutyrated | 1513 | MS, LRI | 0.92±0.05 | 1.10±0.03 | 0.98±0.01 | 0.83±0.03 | 0.75±0.01 |
| Caryophyllene oxide | 1571 | MS, LRI, std | 0.33±0.01 | 0.18±0.00 | 0.27±0.02 | 0.36±0.01 | 0.39±0.00 |
| Humulene oxided | 1597 | MS, LRI | 0.38±0.05 | 0.29±0.00 | 0.31±0.01 | 0,41±0.01 | 0.40±0.01 |
| Monoterpenes | 31.3 | 48.8 | 62.1 | 46.3 | 53.1 | ||
| Alcohols | 15.8 | 14.0 | 15.1 | 17.0 | 15.5 | ||
| Esters | 39.9 | 24.3 | 9.0 | 26.6 | 22.3 | ||
| Ethers | 2.1 | 4.3 | 2.2 | 1.7 | |||
| Sesquiterpenes | 3.8 | 3.6 | 3.3 | 2.4 | 2.4 | ||
| Oxides | 0.7 | 0.7 | 0.9 | 0.8 | 0.8 | ||
| Identified compounds | 93.4 | 94.7 | 94.4 | 94.9 | 95.4 | ||
| Componentsa | LRIb | ID Methodc | Composition (%)±SDe | ||||
|---|---|---|---|---|---|---|---|
| May | June | July | September | October | |||
| Tricyclened | 908 | MS, LRI | 0.01±0.00 | 0.01±0.00 | 0.02±0.00 | ||
| α-Thujened | 917 | MS, LRI | 0.05±0.00 | 0.03±0.00 | 0.12±0.00 | ||
| α-Pinene | 921 | MS, LRI, std | 6.55±0.01 | 4.22±0.22 | 8.81±0.61 | 2.78±0.77 | |
| Camphened | 937 | MS, LRI | 0.01±0.00 | 0.01±0.00 | 0.05±0.00 | ||
| β-Pinene | 963 | MS, LRI, std | 0.11±0.00 | 0.08±0.00 | 0.34±0.01 | ||
| Myrcene | 986 | MS, LRI, std | 0.08±0.00 | 0.08±0.02 | 0.46±0.02 | ||
| δ-3-Carened | 998 | MS, LRI | 0.05±0.00 | 0.04±0.01 | 0.16±0.00 | ||
| α-Terpinene | 1003 | MS, LRI, std | 0.09±0.00 | 0.08±0.01 | 0.25±0.00 | ||
| p-Cymene | 1015 | MS, LRI, std | 0.07±0.00 | 0.03±0.00 | 0.82±0.05 | ||
| Limonene+1,8-Cineole | 1022 | MS, LRI, std | 11.85±0.00 | 12.04±0.31 | 20.40±2.54 | 10.58±0.77 | |
| o-Cymened | 1036 | MS, LRI | 0.01±0.00 | 0.03±0.00 | 0.03±0.00 | ||
| γ-Terpinene | 1043 | MS, LRI, std | 0.25±0.00 | 0.09±0.00 | 1.19±0.07 | ||
| Linalool oxide | 1051 | MS, LRI, std | 0.14±0.00 | 0.08±0.01 | 0.26±0.00 | ||
| α-Terpinolened | 1081 | MS, LRI | 0.22±0.00 | 0.12±0.00 | 0.20±0.01 | ||
| Linalool | 1097 | MS, LRI, std | 4.59±0.01 | 10.47±0.18 | 4.24±0.14 | 3.42±0.52 | |
| Fenchol | 1100 | MS, LRI, std | 0.71±0.03 | 0.48±0.07 | 0.72±0.10 | ||
| Trans-pinocarveold | 1129 | MS, LRI | 0.09±0.02 | 0.10±0.00 | 0.18±0.00 | ||
| Borneol | 1160 | MS, LRI, std | 0.21±0.00 | 0.19±0.00 | 0.27±0.01 | ||
| Terpinen-4-ol | 1169 | MS, LRI, std | 0.28±0.02 | 0.32±0.01 | 0.70±0.03 | ||
| α-Terpineol | 1184 | MS, LRI, std | 3.38±0.01 | 5.76±0.08 | 2.70±0.20 | 2.33±0.41 | |
| Myrtenol | 1189 | MS, LRI, std | 44.03±7.00 | 3.05±0.00 | 8.38±0.21 | 6.78±0.77 | 12.41±2.02 |
| Geraniol | 1255 | MS, LRI, std | 1.16±0.00 | 2.83±0.06 | 1.85±0.07 | 3.17±0.08 | |
| Borneol acetate | 1282 | MS, LRI, std | 0.02±0.00 | 0.04±0.00 | 0.09±0.03 | 0.76±0.02 | |
| Trans pinocarvyl acetated | 1292 | MS, LRI | 0.71±0.03 | 0.56±0.00 | 0.55±0.03 | 0.76±0.02 | |
| Myrtenyl acetate | 1322 | MS, LRI, std | 23.97±6.89 | 33.55±0.08 | 24.62±0.21 | 21.97±0.64 | 26.25±4.03 |
| α-Terpenyl acetated | 1337 | MS, LRI | 1.47±0.01 | 1.53±0.00 | 0.95±0.08 | 0.62±0.38 | |
| Eugenold | 1343 | MS, LRI, std | 1.66±0.00 | 1.49±0.01 | 1.23±0.75 | ||
| Neryl acetate | 1364 | MS, LRI, std | 0.21±0.01 | 0.05±0.00 | 0.24±0.01 | ||
| Geranyl acetate | 1377 | MS, LRI, std | 0.43±0.00 | 0.23±0.01 | 0.18±0.00 | ||
| Methyl eugenold | 1383 | MS, LRI, std | 5.16±0.01 | 3.66±0.01 | 4.93±0.24 | 6.53±0.24 | |
| β-Caryophyllene | 1404 | MS, LRI, std | 5.34±0.02 | 4.48±0.09 | 2.85±0.09 | 2.56±088 | |
| α-Humulene | 1442 | MS, LRI, std | 19.12±5.02 | 1.57±0.00 | 0.66±0.00 | 1.48±0.08 | 0.88±0.31 |
| Geranyl isobutyrated | 1513 | MS, LRI | 0.85±0.03 | 0.88±0.01 | 0.10±0.01 | ||
| Caryophyllene oxide | 1573 | MS, LRI, std | 0.66±0.00 | 0.88±0.03 | 1.67±0.16 | 6.04±0.22 | |
| Humulene oxided | 1599 | MS, LRI, std | 0.84±0.07 | 0.92±0.09 | 1.29±0.13 | 2.00±0.81 | |
| Monoterpenes | 19.3 | 16.8 | 32.8 | 13.4 | |||
| Alcohols | 44 | 15.1 | 30.1 | 18.7 | 20.9 | ||
| Esters | 24 | 37.2 | 27.91 | 24.1 | 27.4 | ||
| Ethers | 5.2 | 3.7 | 4.9 | 7.0 | |||
| Sesquiterpenes | 19 | 7.6 | 6.0 | 6.0 | 9.5 | ||
| Oxides | 1.6 | 1.9 | 3.2 | 8.0 | |||
| Identified compounds | 87 | 85.4 | 83.4 | 88.1 | 80.2 | ||
| Componentsa | LRIb | ID Methodc | Composition (%)±SDe | |
|---|---|---|---|---|
| September | October | |||
| Tricyclened | 911 | MS, LRI | 0.02±0.00 | 0.05±0.00 |
| α- Thujened | 919 | MS, LRI | 0.023±0.00 | 0.04±0.00 |
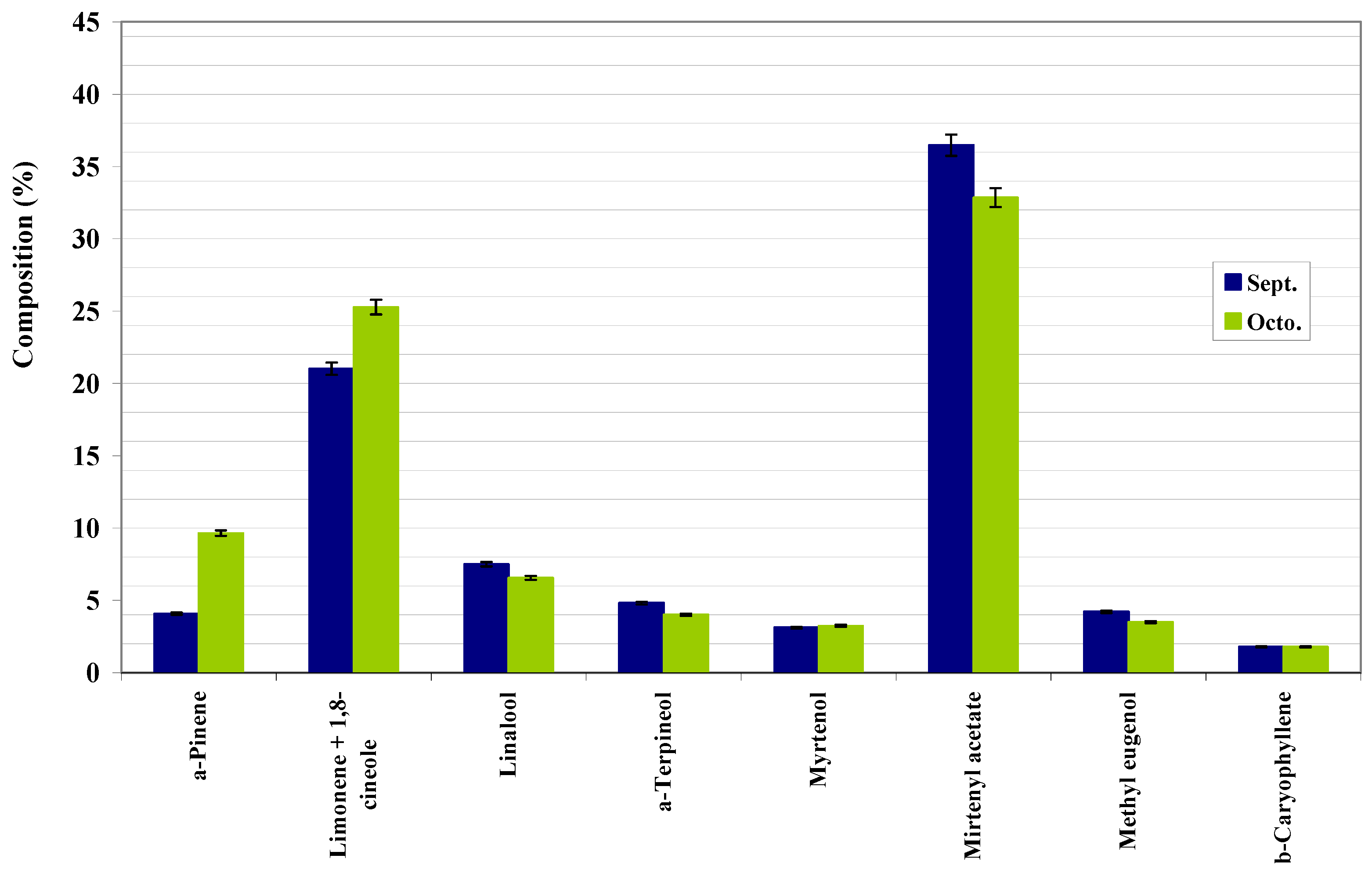
| α-pinene | 923 | MS, LRI, std | 4.08±0.36 | 9.65±0.02 |
| Camphened | 943 | MS, LRI | 0.01±0.00 | 0.01±0.00 |
| β-Pinene | 965 | MS, LRI, std | 0.09±0.01 | 0.17±0.00 |
| Myrcene | 992 | MS, LRI, std | 0.04±0.02 | 0.07±0.01 |
| δ-3-Carened | 1003 | MS, LRI | 0.14±0.01 | 0.02±0.01 |
| α-Terpinene | 1010 | MS, LRI, std | 0.01±0.01 | 0.13±0.01 |
| p-cymene | 1020 | MS, LRI, std | 0.11±0.01 | 0.11±0.00 |
| Limonene+1,8-cineole | 1025 | MS, LRI, std | 21.02±0.88 | 25.28±0.09 |
| o-Cymened | 1040 | MS, LRI | 0.01±0.00 | |
| γ-Terpinene | 1053 | MS, LRI, std | 0.21±0.01 | 0.16±0.01 |
| Linalool oxide | 1071 | MS, LRI, std | 0.02±0.00 | 0.02±0.00 |
| α-Terpinolened | 1083 | MS, LRI, | 0.14±0.01 | 0.07±0.01 |
| Linalool | 1098 | MS, LRI, std | 7.50±0.33 | 6.56±0.07 |
| Fenchol | 1102 | MS, LRI, std | 0.57±0.04 | 0.65±0.03 |
| Trans pinocarveold | 1130 | MS, LRI | 0.13±0.03 | 0.17±0.04 |
| Borneol | 1162 | MS, LRI, std | 0.13±0.01 | 0.10±0.01 |
| Terpinen-4-ol | 1171 | MS, LRI, std | 0.38±0.02 | 0.26±0.00 |
| α-Terpineol | 1186 | MS, LRI, std | 4.81±0.22 | 4.01±0.01 |
| Myrtenol | 1191 | MS, LRI, std | 3.11±0.15 | 3.24±0.02 |
| Geraniol | 1255 | MS, LRI, std | 1.02±0.06 | 0.93±0.00 |
| Borneol acetate | 1280 | MS, LRI, std | 0.02±0.00 | 0.06±0.07 |
| Trans pinocarvyl acetated | 1294 | MS, LRI | 0.46±0.39 | 0.64±0.00 |
| Myrtenyl acetate | 1327 | MS, LRI, std | 36.48±1.48 | 32.86±0.21 |
| α-Terpenyl acetated | 1338 | MS, LRI | 0.81±0.03 | 0.54±0.00 |
| Eugenold | 1346 | MS, LRI | 1.19±0.04 | 0.26±0.00 |
| Neryl acetate | 1369 | MS, LRI, std | 0.07±0.01 | 0.07±0.00 |
| Geranyl acetated | 1379 | MS, LRI, std | 0.24±0.01 | 0.19±0.00 |
| Methyl eugenol | 1384 | MS, LRI | 4.22±0.15 | 3.50±0.03 |
| β-Caryophyllene | 1404 | MS, LRI, std | 1.79±0.05 | 1.79±0.04 |
| α-Humulene | 1445 | MS, LRI, std | 0.56±0.03 | 0.34±0.00 |
| β-Selinened | 1478 | MS, LRI | 0.17±0.01 | 0.13±0.00 |
| α-Selinened | 1487 | MS, LRI | 0.18±0.01 | 0.12±0.01 |
| Geranyl isobutyrated | 1513 | MS, LRI | 0.35±0.01 | 0.319±0.004 |
| Caryophyllene oxide | 1574 | MS, LRI, std | 0.58±0.03 | 0.669±0.007 |
| Humulene oxided | 1601 | MS, LRI, std | 0.61±0.03 | 0.61±0.03 |
| Monoterpenes | 25.9 | 35.8 | ||
| Alcohols | 18.8 | 16.2 | ||
| Esters | 38.4 | 34.7 | ||
| Ethers | 4.2 | 3.5 | ||
| Sesquiterpenes | 3.3 | 3.0 | ||
| Oxides | 1.2 | 1.3 | ||
| Identified compounds | 91.3 | 93.9 | ||

| May | June | July | September | September | October | October | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| leaves | leaves | leaves | leaves | berries | leaves | berries | ||||||||||||||||||||||
| α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | Linalool | α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | linalool | α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | linalool | α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | linalool | α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | linalool | α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | linalool | α-pinene | 1,8-cineole+limonene | Myrtenyl acetate | linalool | |
| Moroccan [10] | 10b | 43.5b | 25b | 0.3b | ||||||||||||||||||||||||
| Italy (Sardinia) [14] | 50.0d | 37.0d | - | 0.2d | 26.4d | 26.8d | - | 2.0d | ||||||||||||||||||||
| Tunisia [12] | 45.9 | 32.1 | ||||||||||||||||||||||||||
| Croatia [9] | 12.2 | 17.5 | 30.7 | 11.6 | 6.6 | 12.6 | 24.9 | 10.8 | 8 | 18.8 | 20.7 | 14.7 | 16.4 | 29.8 | 20.8 | 18.3 | 7.4 | 18.4 | 5.5 | 13.2 | 15.9 | 26.6 | 12.6 | 12.7 | 10.9 | 4.7 | ||
| Greece [7] | 18b | 21.8bc | 1.1b | |||||||||||||||||||||||||
| Turkey [6] | 6.4b | 21.6b | 14.5b | 16.3b | ||||||||||||||||||||||||
| Albania (Div) [5] | 19.4 | 32.7 | 11.4 | 8.79 | ||||||||||||||||||||||||
| Albania(Elba) [5] | 20.3 | 29 | 12.3 | 13.4 | ||||||||||||||||||||||||
| France (Corsica) [3] | 57.2a | 26.4a | 1.6a | 53a | 23a | 2.3a | 51.3a | 26.8a | 2.5a | 56.9a | 22.8a | 2.1a | 47a | 26.6a | 2.4a | |||||||||||||
| Spain [4] | 8.18 | 37.5 | 35.9 | 0.05 | ||||||||||||||||||||||||
| Tunisia[13] | 7.2 | 41.0 | 1.43 | 18.9 | 7.5 | 18.4 | 0.26 | 3.2 | ||||||||||||||||||||
| Portugal (this work) | 10.4 | 20 | 37.6 | 7.6 | 16.5 | 31.3 | 22.2 | 7.0 | 21.5 | 39.5 | 7.4 | 6.2 | 13.1 | 32.1 | 24.8 | 9.3 | 4.1 | 2 | 7.5 | 36.5 | 15.3 | 36.8 | 20.8 | 7.9 | 9.7 | 25.9 | 6.6 | 32.9 |
3. Experimental
3.1. Plant material
3.2. Chemicals
3.3. Oil isolation
3.4. GC-FID analysis
3.5. GC-MS analysis
3.6. Statistical analysis
4. Conclusions
Acknowledgements
References
- Elfellah, M.S.; Akhter, M.H.; Khan, M.T. Antihyperglycaemic effect of an extract of Myrtus communis in Streptozotocin-induced diabetes in mice. J. Ethnopharmacol. 1984, 11, 275–281. [Google Scholar] [CrossRef]
- Chalchat, J.; Garry, R.; Michet, A. Essential Oils of Myrtle of the Mediterrenean Litoral. J. Essent. Oil Res. 1998, 10, 613–617. [Google Scholar] [CrossRef]
- Bradesi, P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A. Chemical Composition of Myrtle Leaf Essential Oil from Corsica (France). J. Essent. Oil Res. 1997, 9, 283–288. [Google Scholar] [CrossRef]
- Boelens, M.; Jimenez, R. The chemical composition of Spanish Myrtle Oils. Part II. J. Essent. Oil Res. 2002, 4, 349–353. [Google Scholar] [CrossRef]
- Asllani, U. Chemical Composition of Albanian Myrtle Oil. J. Essent. Oil Res. 2000, 12, 140–142. [Google Scholar] [CrossRef]
- Ozek, T.; Demirci, B.; Baser, K. Chemical Composition of Tukish Myrtle Oil. J. Essent. Oil Res. 2000, 12, 541–544. [Google Scholar] [CrossRef]
- Koukos, P.K.; Papadopoulou, K.I.; Papagiannopoulos, A.D.; Patiaka, D.T. Chemicals from Greek forestry Biomass: Constituents of the leaf Oil of Myrtus communis L. Grown in Greece. J. Essent. Oil Res. 2001, 13, 245–256. [Google Scholar] [CrossRef]
- Gardeli, C.; Papageorgiou, V.; Mallouchos, A.; Theodosis, K.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Jerkovic, I.; Radonic, A.; Borcic, I. Comparative Study of Leaf, Fruit and Flower Essential Oil of Croatian Myrtus communis During a One Year Vegetative Cycle. J. Essent. Oil Res. 2002, 14, 266–270. [Google Scholar] [CrossRef]
- Farah, A.; Afifi, A.; Fechtal, M.; Chhen, A.; Satrani, B.; Talbi, M.; Chaouch, A. Fractional distillation effect on the chemical composition of Moroccan myrtle essential oils. Flav. Frag. J. 2006, 21, 351–354. [Google Scholar] [CrossRef]
- Messaoud, C.; Zaouali, Y.; Salah, A.; Khoudja, M.; Boussaid, M. Myrtus communis in Tunisia: variability of the essential oil composition in natural populations. Flav. Frag. J. 2005, 20, 577–582. [Google Scholar] [CrossRef]
- Jamoussi, B.; Romdhane, M.; Abderraba, A.; Hassine, B.; Gadri, A. Effect of harvest time on the yield and composition of Tunisian myrtle oils. Flav. Frag. J. 2005, 20, 274–277. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Marzouk, B. Variations in essential oil and fatty acid composition during Myrtus communis var. italica fruit maturation. Food Chem. 2009, 112, 621–626. [Google Scholar] [CrossRef]
- Tuberoso, C.; Barra, A.; Angioni, A.; Sarritzu, E.; Pirisi, F. Chemical composition of volatiles in Sardinian Myrtle (Myrtus communis L.) alcoholic extracts and essential oils. J. Agric. Food Chem. 2006, 54, 1420–1426. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Melis, M.; Angioni, A.; Pala, M.; Cabras, P. Myrtle hydroalcoholic extracts obtained from different selections of Myrtus communis L. Food Chem. 2007, 101, 806–811. [Google Scholar] [CrossRef]
- Rao, B.R.R.; Kaul, P.N.; Syamasundar, K.V.; Ramesh, S. Chemical profiles of primary and secondary essential oils of palmarosa (Cymbopogon martini (Roxb.) Wats var motia Burk). Ind. Crop Prod. 2005, 21, 121–127. [Google Scholar] [CrossRef]
- Zellner, B.; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention in gas chromatographic analysis: a review. Flav. Frag. J. 2008, 23, 297–314. [Google Scholar] [CrossRef]
- Babushok, V.; Zenkevich, I. Retention Indices for most frequently reported essential Oil Compounds in GC. Chomatographia 2009, 69, 257–269. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, P.C.; Cebola, M.-J.; Bernardo-Gil, M.G. Evolution of the Yields and Composition of Essential Oil from Portuguese Myrtle (Myrtus comunis L.) through the Vegetative Cycle. Molecules 2009, 14, 3094-3105. https://doi.org/10.3390/molecules14083094
Pereira PC, Cebola M-J, Bernardo-Gil MG. Evolution of the Yields and Composition of Essential Oil from Portuguese Myrtle (Myrtus comunis L.) through the Vegetative Cycle. Molecules. 2009; 14(8):3094-3105. https://doi.org/10.3390/molecules14083094
Chicago/Turabian StylePereira, Paula C., Maria-João Cebola, and M. Gabriela Bernardo-Gil. 2009. "Evolution of the Yields and Composition of Essential Oil from Portuguese Myrtle (Myrtus comunis L.) through the Vegetative Cycle" Molecules 14, no. 8: 3094-3105. https://doi.org/10.3390/molecules14083094




