Enantioselective, Organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman Reactions: Stereochemical Issues
Abstract
:1. Introduction

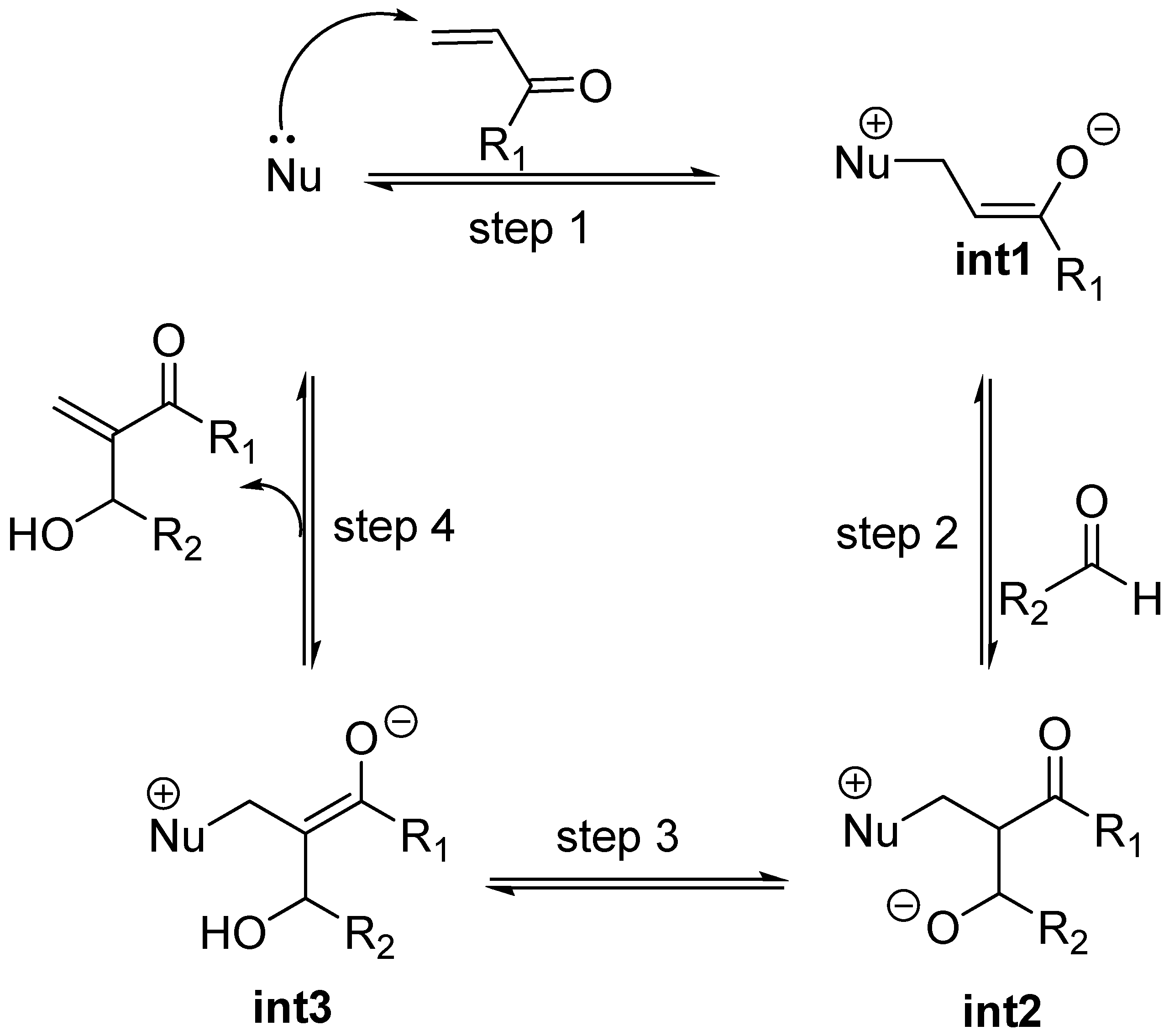


2. The Standard MBH and Aza-MBH Reactions


 ) and aza-MBH (
) and aza-MBH (  ) reactions.
) reactions.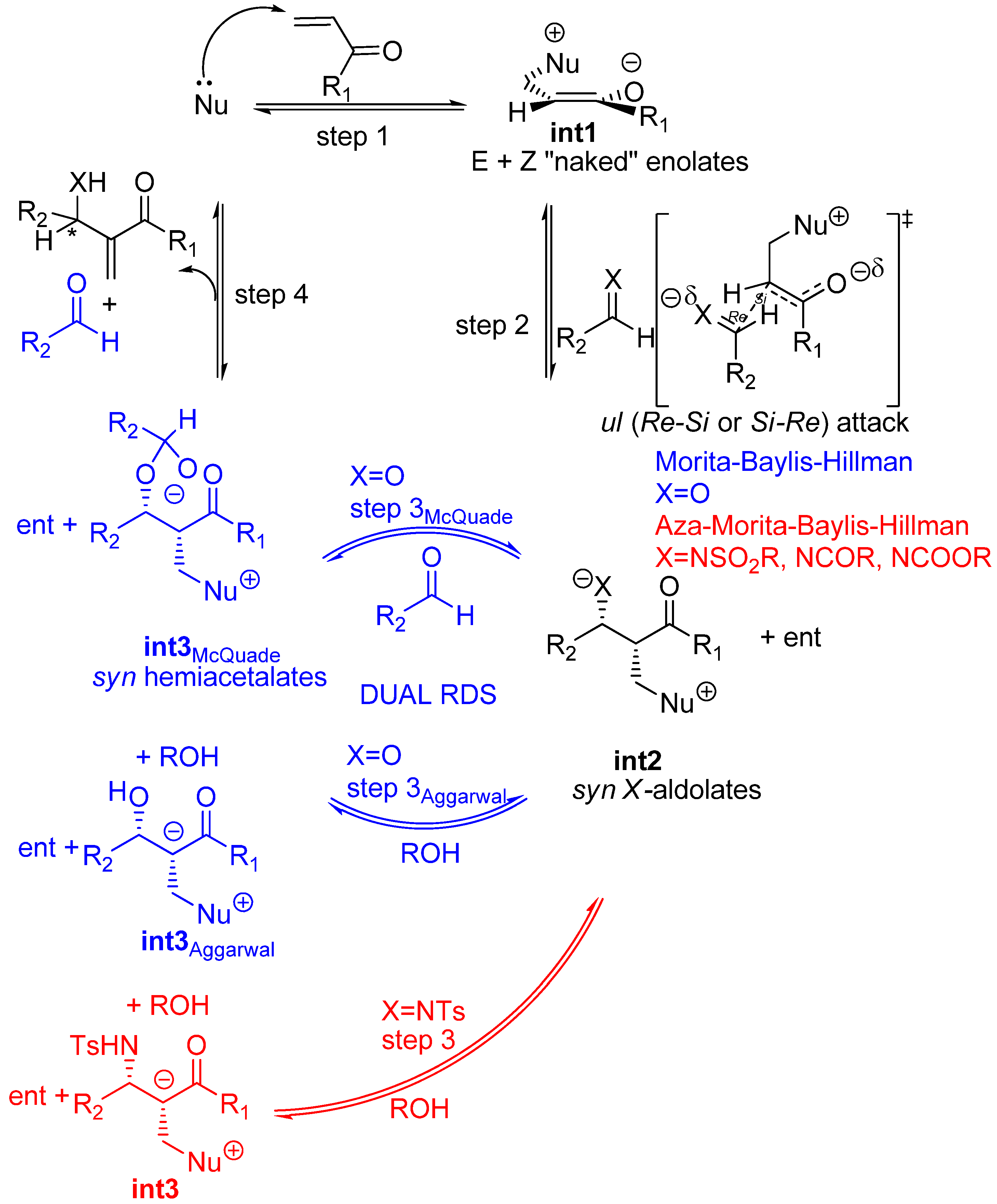


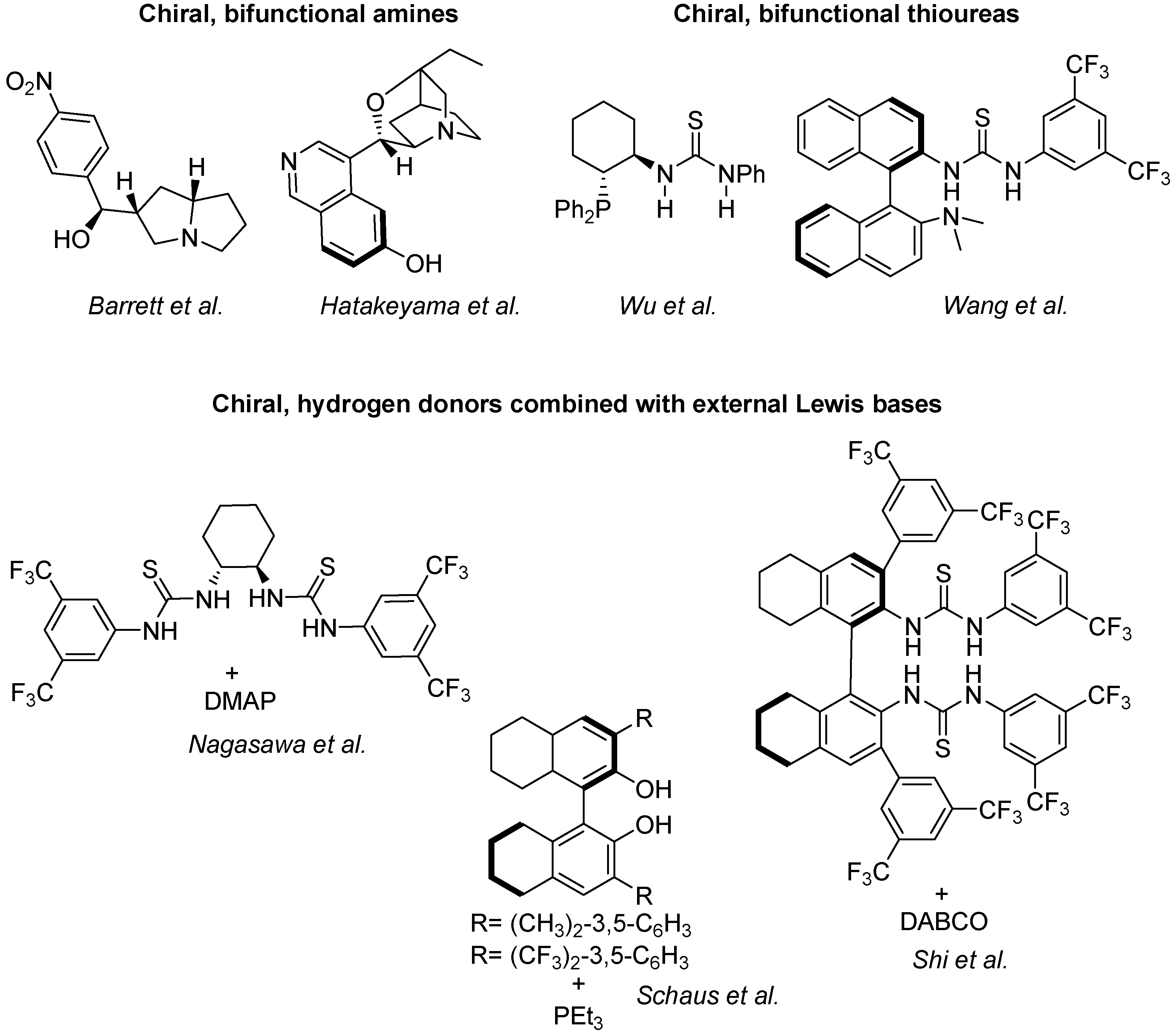

3. The α-Aminoacid Catalyzed and α-Aminoacid-Amine Cocatalyzed Mbh and Aza-Mbh Reactions






4. Conclusions
Acknowledgements
References and Notes
- Morita, K.; Suzuki, Z.; Hirose, H. A Tertiary Phosphine-catalyzed Reaction of Acrylic Compounds with Aldehydes. Bull. Chem Soc. Jpn. 1968, 41, 2815–2815. [Google Scholar] [CrossRef]
- Baylis, A.B.; Hillman, M.E.D. Ger. Offen. 2, 155,133 (1972). Acrylic compounds. US Pat. 3,743,669, 1973. Chem. Abstr. 1972, 77, 34174. [Google Scholar]
- Perlmutter, P.; Teo, C.C. A simple synthesis of 2-methyledene-3-aminopropanoates. Tetrahedron Lett. 1984, 25, 5951–5952. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. Enantioselective Organocatalysis. Angew. Chem. Int. Ed. 2001, 40, 3726–3748. [Google Scholar] [CrossRef]
- Jarvo, E.R.; Miller, S.J. Amino acids and peptides as asymmetric organocatalysts. Tetrahedron 2002, 58, 2481–2495. [Google Scholar] [CrossRef]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-Supported Organic Catalysts. Chem. Rev. 2003, 103, 3401–3430. [Google Scholar] [CrossRef]
- Berkessel, A.; Gröger, H. Metal-Free Organic Catalysts in Asymmetric Synthesis; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Dalko, P.I.; Moisan, L. In the Golden Age of Organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Seayad, J.; List, B. Asymmetric Organocatalysis. Org. Biomol. Chem. 2005, 3, 719–724. [Google Scholar] [CrossRef]
- Marcelli, T.; vanMaarseveen, J.H.; Hiemstra, H. Cupreines and Cupreidines: An Emerging Class of Bifunctional Cinchona Organocatalysts. Angew. Chem. Int. Ed. 2006, 45, 7496–7504. [Google Scholar] [CrossRef]
- Shi, M.; Xu, Y.-M.; Zhao, G.-L.; Wu, X.-F. Lewis Base Effects in the Baylis-Hillman Reaction of Arenecarbaldehydes and N-Aryliden-4-methylbenzenesolfonamides with α-β-Unsaturated Cyclic Ketones. Eur. J. Org. Chem. 2002, 3666–3679. [Google Scholar]
- Shi, M.; Xu, Y.-M. Lewis base effects in the Baylis-Hillman Reaction of imines with methyl vinyl ketone. Eur. J. Org. Chem. 2002, 4, 696–701. [Google Scholar]
- Aggarwal, V.K.; Emme, S.Y.; Fulford, S.Y. Correlation between pKa and reactivity of quinuclidine-based catalysts in the Baylis-Hillman Reaction: discovery of quinuclidine as optimum catalyst leading to substantial enhancement of scope. J. Org. Chem. 2003, 68, 692–700. [Google Scholar]
- Aggarwal, V.K.; Mereu, A. Superior amine catalysts for the Baylis-Hillman reaction: the use of DBU and its implications. Chem. Commun. 1999, 2311–2312. [Google Scholar] [CrossRef]
- Rezgui, F.; El Gaied, M.M. DMAP-catalyzed hydroxymethylation of 2-cyclohexenone in aqueous medium through Baylis-Hillman reaction. Tetrahedron Lett. 1998, 39, 5965–5966. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, B.; He, J.; Janczuk, A.; Wang, P.G.; Cheng, J. Aqueous Baylis-Hillman reaction of cyclopent-2-enone using imidazole as catalyst. Tetrahedron Lett. 2002, 43, 7369–7371. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Van der Pol, C. Development of catalysts for the Baylis-Hillman reaction: the application of tetramethylguanidine and attempts to use a supported analogue. J. Chem. Soc. Perkin Trans. 2001, 1, 2831–2835. [Google Scholar] [CrossRef]
- He, L.; Jian, T.-Y.; Ye, S. N-Heterocyclic Carbene Catalyzed aza-Morita-Baylis-Hillman Reaction of Cyclic Enones with N-Tosylarylimines. J. Org. Chem. 2007, 72, 7466–7468. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.-R.; Huang, X.-L.; Ye, S. Chiral Bifunctional N-Heterocyclic Carbenes: Synthesis and Application in the aza-Morita-Baylis-Hillman Reaction. Synthesis 2008, 2825–2829. [Google Scholar]
- Oda, R.; Kawabata, T.; Tanimoto, S. Entstehung von P-Ylid aus Triphenylphosphin und Acrylsäurederivaten. Tetrahedron Lett. 1964, 5, 1653–1657. [Google Scholar] [CrossRef]
- For a recent review: Methot, J.L.; Roush, W.R. Nucleophilic phosphine organocatalysis. Adv. Synth. Catal. 2004, 346, 1035–1050. [Google Scholar] [CrossRef]
- Denmark, S.E.; Gregory, L.B. Lewis Base Catalysis in Organic Synthesis. Angew. Chem. Int. Ed. 2008, 47, 1560–1638. [Google Scholar] [CrossRef]
- Drewes, S.E.; Roos, G.H.P. Synthetic potential of the tertiary-amine-catalyzed reaction of activated vinyl carbanions with aldehydes. Tetrahedron 1988, 44, 4653–4670. [Google Scholar] [CrossRef]
- Basavaiah, P.D. Rao; Hyma, R.S. The Baylis−Hillman reaction: a novel carbon-carbon forming reaction. Tetrahedron 1996, 52, 8001–8062. [Google Scholar] [CrossRef]
- Ciganek, E. Organic Reactions; John Wiley & sons, Inc.: New York, NY, USA, 1997; Volume 51, p. 201. [Google Scholar]
- Langer, P. New strategies for the development of an asymmetric version of the Baylis-Hillman reaction. Angew. Chem. Int. Ed. 2000, 39, 3049–3052. [Google Scholar] [CrossRef]
- Basavaiah, D.; Rao, A.J.; Satyanarayana, T.T. Recent advances in the Baylis−Hillman Reaction and Applications. Chem. Rev. 2003, 103, 811–892. [Google Scholar] [CrossRef]
- Basavaiah, D.; Rao, K.V.; Reddy, R.J. The Baylis–Hillman reaction: a novel source of attraction, opportunities, and challenges in synthetic chemistry. Chem. Soc. Rev. 2007, 36, 1581–1588. [Google Scholar] [CrossRef]
- Singh, V.; Batra, S. Advances in the Baylis-Hillman reaction-assisted synthesis of cyclic frameworks. Tetrahedron 2008, 64, 4511–4574. [Google Scholar] [CrossRef]
- Keck, G.E.; Welch, D.S. Intramolecular Baylis-Hillman and Morita Reactions Using Unsaturated Thiol Ester Substrates Containing Enolizable Aldehydes. Org. Lett. 2002, 4, 3687–3690. [Google Scholar] [CrossRef]
- Yagi, K.; Turitani, T.; Shinokubo, H.; Oshima, K. Intramolecular Tamdem Michael-Type Addition/Aldol Cyclization Induced by TiCl4/R4NX Combinations. Org. Lett. 2002, 4, 3111–3114. [Google Scholar] [CrossRef]
- Shi, Y.-L.; Shi, M. Aza-Baylis-Hillman reactions and their synthetic applications. Eur. J. Org. Chem. 2007, 2905–2916. [Google Scholar]
- Masson, G.; Housseman, C.; Zhu, J. The enantioselective Morita−Baylis−Hillman reaction and its aza counterpart. Angew. Chem. Int. Ed. 2007, 46, 4614–4628. [Google Scholar] [CrossRef]
- Declerck, V.; Martinez, J.; Lamaty, F. Aza-Baylis−Hillman Reaction. Chem. Rev. 2009, 109, 1–48. [Google Scholar] [CrossRef]
- Ma, G.-N.; Jiang, J.-J.; Shi, M.; Wei, Y. Recent extensions of the Morita-Baylis-Hillman reaction. Chem. Commun. 2009, 5496–5514. [Google Scholar]
- Hill, J.S.; Isaacs, N.S. Mechanism of substitution reactions of acrylic derivatives. J. Phys. Org. Chem. 1990, 3, 285–288. [Google Scholar]
- Menozzi, C.; Dalko, P.I. Enantioselective Organocatalysis: Reactions and Experimental Procedures; Wiley-VCH: Weinheim, Germany, 2004; pp. 151–187. [Google Scholar]
- Hoffman, H.R.M.; Rabe, J. A new, efficient and stereocontrolled synthesis of trisubstituted alkenes via functionalized acrylic esters. Angew. Chem. Int. Ed. 1983, 22, 796–797. [Google Scholar] [CrossRef]
- Bode, M.L.; Kaye, P.T. A kinetic and mechanistic study of the Baylis−Hillman reaction. Tetrahedron Lett. 1991, 32, 5611–5614. [Google Scholar] [CrossRef]
- Fort, Y.; Berthe, M.C.; Caubère, P. The Baylis−Hillman reaction-Mechanism and applications revisited. Tetrahedron 1992, 48, 6371–6384. [Google Scholar] [CrossRef]
- Rozendaal, E.M.L.; Voss, B.M.W.; Scheeren, H.W. Effect of solvent, pressure and catalyst on the E/Z selectivity in the Baylis-Hillman reaction between crotononitrile and benzaldehyde. Tetrahedron 1993, 49, 6931–6936. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.-H.; Li, C.-Q. Chiral phosphine Lewis bases catalyzed asymmetric aza-Baylis-Hillman reaction of N-sulfonated imines with activated olefins. J. Am. Chem. Soc. 2005, 127, 3790–3800. [Google Scholar] [CrossRef]
- Santos, L.S.; Pavam, C.H.; Almeida, W.P.; Coelho, F.; Eberlin, M.N. Probing the mechanism of the Baylis-Hillman reaction by electrospray ionization mass and tandem mass spectrometry. Angew. Chem. Int. Ed. 2004, 43, 4330–4333. [Google Scholar] [CrossRef]
- Raheem, I.T.; Jacobsen, E.N. Highly Enantioselective Aza-Baylis-Hillman Reactions Catalyzed by Chiral Thiourea Derivatives. Adv. Synth. Catal. 2005, 347, 1701–1708. [Google Scholar] [CrossRef]
- Byun, H.S.; Reddy, K.C.; Bittman, R. Improved syntheses of ethyl(α-bromomethyl)acrylate and 2-methylene-1,3-propanediol via ethyl α-(hydroxymethyl) acrylate. Tetrahedron Lett. 1994, 35, 1371–1374. [Google Scholar]
- Auge, J.; Lubin, N.; Lubineau, A. Acceleration in water of the Baylis-Hillman reaction. Tetrahedron Lett. 1994, 35, 7947–7948. [Google Scholar]
- Basavaiah, D.; Krishnamacharyulu, M.; Rao, A.J. The Aqueous Trimethylamine Mediated Baylis-Hillman Reaction. Synth. Commun. 2000, 30, 2061–2069. [Google Scholar] [CrossRef]
- Yu, C.Z.; Liu, B.; Hu, L.Q. Efficient Baylis-Hillman Reaction Using Stoichiometric Base Catalyst and an Aqueous Medium. J. Org. Chem. 2001, 66, 5413–5418. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, Z.; Zhao, G.; Tang, C. Dramatic Rate Acceleration of the Baylis-Hillman Reaction in Homogeneous Medium in the Presence of Water. Org. Lett. 2002, 4, 4723–4725. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, J.; Choo, H.; Chong, Y. Octanol-Accelerated Baylis-Hillman Reaction. Synlett. 2007, 395–398. [Google Scholar]
- Shi, M.; Liu, Y.H. Traditional Morita-Baylis-Hillman reaction of aldehydes with methyl vinyl ketone co-catalyzed with triphenylphosphine and nitrophenol. Org. Biomol. Chem. 2006, 4, 1468–1470. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Cook, A.S.; Kamimura, A. Asymmetric Baylis-Hillman reaction: catalysis using a chiral pirrolizidine base. Chem. Commun. 1998, 2533–2534. [Google Scholar]
- Drewes, S.E.; Freese, S.D.; Emslie, N.D.; Roos, G.H.P. Synthesis of 3-Hydroxy-2-Methylene Carbonyl Compounds. Synth. Commun. 1988, 18, 1565–1572. [Google Scholar] [CrossRef]
- Bailey, M. ; Markó, I.E.; Ollis, W.D.; Rasmussen, P.R. Stereoselective epoxidation of hydroxyenones. The synthesis of the sidechain of clerocidin. Tetrahedron Lett. 1990, 31, 4509–4512. [Google Scholar] [CrossRef]
- Kawahara, S.; Nakano, A.; Esumi, T.; Iwabuchi, Y.; Hatakeyama, S. Isocupreidine-catalyzed Asymmetric Baylis-Hillman Reaction of Imines. Org. Lett. 2003, 5, 3103. [Google Scholar] [CrossRef]
- Shi, M. Catalytic, Asymmetric Baylis-Hillman Reaction of Imines with Methyl Vinyl Ketone and Methyl Acrylate. Angew. Chem. Int. Ed. 2002, 41, 4507. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Y.; Chen, R. A Novel Selective Aza-Morita-Baylis-Hillman (aza-MBH) Domino Reaction and aza-MBH Reaction of N-Sulfonyl Imines with Acrolein Catalyzed by Bifunctional Phosphine Organocatalyst. Chem. Eur. J. 2008, 14, 6852–6856. [Google Scholar] [CrossRef]
- Matsui, K.; Takizawa, S. A Brönsted acid and Lewis base organocatalyst for the aza-Morita–Baylis–Hillman reaction. Synlett. 2006, 761–765. [Google Scholar]
- Price, K.E.; Broadwater, S.J.; Jung, H.M.; McQuade, D.T. Baylis−Hillman mechanism: A New Interpretation in Aprotic Solvents. Org. Lett. 2005, 7, 147–150. [Google Scholar]
- Price, K.E.; Broadwater, S.J.; Walker, B.J.; McQuade, D.T. A new interpretation of the Baylis−Hillman mechanism. J. Org. Chem. 2005, 70, 3980–3987. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Fulford, F.Y.; Lloyd-Jones, G.C. Re-evaluation of the mechanism of the Baylis−Hillman reaction - implications for asymmetric catalysis. Angew. Chem. Int. Ed. 2005, 44, 1706–1708. [Google Scholar] [CrossRef]
- Robiette, R.; Aggarwal, V.K.; Harvey, J.N. Mechanism of the Morita−Baylis−Hillman reaction: A computational investigation. J. Am. Chem. Soc. 2007, 129, 15513–15525. [Google Scholar] [CrossRef]
- Amarante, G.W.; Milagre, H.M.S.; Vaz, B.G.; Ferreira, B.R.V.; Eberlin, M.C.; Coelho, F. Dualistic nature of the mechanism of the Morita-Baylis-Hillman reaction probed by electrospray ionization mass spectrometry. J. Org. Chem. 2009, 74, 3031–3037. [Google Scholar]
- Carrasco-Sanchez, V.; Simirgiotis, M.J.; Santos, L.S. The Morita-Baylis-Hillman reaction: insights into asymmetry and reaction mechanisms by electrospray ionization mass spectrometry. Molecules 2009, 14, 3989–4021. [Google Scholar] [CrossRef]
- Online mechanistic investigations of catalyzed reactions by electrospray ionization mass spectrometry: A tool to intercept transient species in solution. Eur. J. Org. Chem. 2008, 235–253.
- Buskens, P.; Klankermayer, J.; Leitner, W. Bifunctional Activation and Racemization in the Catalytic Asymmetric aza-Baylis-Hillman Reaction. J. Am. Chem. Soc. 2005, 127, 16762–16763. [Google Scholar] [CrossRef]
- Utsumi, N.; Zhang, H.; Tanaka, F.; Barbas, C.B., III. A Way to Highly Enantiomerically Enriched aza-Morita-Baylis-Hillman-Type Products. Angew. Chem. Int. Ed. 2007, 46, 1878–1880. [Google Scholar] [CrossRef]
- Aroyan, C.E.; Vasbinder, M.M.; Miller, S.J. Dual Catalyst Control in the Enantioselective Intramolecular Morita-Baylis-Hillman Reaction. Org. Lett. 2005, 7, 3849–3851. [Google Scholar] [CrossRef]
- Miller, S.J. In Search of Peptide-Based Catalysts for Asymmetric Organic Synthesis. Acc. Chem. Res. 2004, 37, 601–610. [Google Scholar] [CrossRef]
- Imbriglio, J.E.; Vasbinder, M.M.; Miller, S.J. Dual Catalyst Control in the Amino Acid-Peptide-Catalyzed Enantioselective Baylis-Hillman Reaction. Org. Lett. 2003, 5, 3741–3743. [Google Scholar] [CrossRef]
- Vasbinder, M.M.; Imbriglio, J.E.; Miller, S.J. Amino acid-peptide-catalyzed enantioselective Morita-Baylis-Hillman reactions. Tetrahedron 2006, 62, 11450–11459. [Google Scholar] [CrossRef]
- Vesely, J.; Dziedzic, P.; Córdova, A. Aza-Morita-Baylis-Hillman-type reactions: highly enantioselective addition of unmodified α,β-unsaturated aldehydes with N-Boc protected imines. Tetrahedron Lett. 2007, 48, 6900–6904. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, J.-K.; Li, C.-Q. Lewis base and L-proline co-catalyzed Baylis-Hillman reaction of arylaldehydes and methyl vinyl ketone. Tetrahedron Lett. 2002, 43, 127–130. [Google Scholar] [CrossRef]
- Chen, S.-H.; Hong, B.-C.; Su, C.-F.; Sarshar, S. An unexpected inversion of enantioselectivity in the proline catalyzed intramolecular Baylis-Hillman reaction. Tetrahedron Lett. 2005, 46, 8899–8903. [Google Scholar] [CrossRef]
- Duarte, F.J.S.; Cabrita, E.J.; Frenking, G.; Santos, A.G. Density functional study of proline-catalyzed intramolecular Baylis-Hillman Reactions. Chem. Eur. J. 2009, 15, 1734–1746. [Google Scholar] [CrossRef]
- Taniguchi, M.; Hino, T.; Kishi, Y. Aldol reactions of allenolates generated by 1,4-addition of iodide anion or its equivalent to acetylenic ketones. Tetrahedron Lett. 1986, 27, 4767–4770. [Google Scholar] [CrossRef]
- Uehira, S.; Nan, Z.; Shinokubo, H.; Oshima, K. Highly Stereoselective coupling reaction of acrolein or vinyl ketone with aldehydes. Org. Lett. 1999, 1, 1383–1385. [Google Scholar] [CrossRef]
- Wei, H.X.; Gao, J.J.; Li, G. Substoichiometric TiCl4-mediated vicinal difunctionalization of α,β-acetylenic ketones for the synthesis of β-halo Baylis-Hillman olefins. Tetrahedron Lett. 2001, 42, 9119–9122. [Google Scholar] [CrossRef]
- Kataoka, T.; Kinoshita, H. Chalcogenide-lewis acid mediated tandem michael aldol reaction. An alternative to the morita-baylis-hillman reaction and a new development. Eur. J. Org. Chem. 2005, 45–48. [Google Scholar] [CrossRef]
- Balan, D.; Adolfsson, H. titanium isopropoxide as efficient catalyst for the Aza-Baylis-Hillman Reaction. selective formation of α-methylene-β-amino acid derivatives. J. Org. Chem. 2002, 67, 2329–2334. [Google Scholar] [CrossRef]
- Walsh, L.M.; Winn, C.L.; Goodman, J.M. Sulfide-BF3.Et2O mediated Baylis-Hillman reactions. Tetrahedron Lett. 2002, 43, 8219–8222. [Google Scholar] [CrossRef]
- Kuwajima, I.; Nakamura, E. Quaternary Ammonium Enolates as Synthetic Intermediates. Regiospecific Alkylation Reaction of Ketones. J. Am. Chem. Soc. 1975, 97, 3257–3258. [Google Scholar] [CrossRef]
- Noyori, R.; Nishida, I.; Sakata, J.; Nishizawa, M. Tris(dimethylamine)sulfonium Enolates. J. Am. Chem. Soc. 1980, 102, 1223–1225. [Google Scholar] [CrossRef]
- Noyori, R.; Nishida, I.; Sakata, J. Alkylation via tris(dialkylamino)sulfonium enolates. Tetrahedron Lett. 1980, 21, 2085–2088. [Google Scholar] [CrossRef]
- Noyori, R.; Yokoyama, K.; Sakata, J.; Kuwajima, I.; Nakamura, E. Fluoride ion catalyzed Aldol Reaction between enol silyl ethers and carbonyl compounds. J. Am. Chem. Soc. 1977, 99, 1265–1267. [Google Scholar] [CrossRef]
- Kleshick, W.A.; Buse, C.T.; Heathcock, C.H. Stereoselection in aldol condensation. J. Am. Chem. Soc. 1977, 99, 247–248. [Google Scholar] [CrossRef]
- Notice that for the case of unsaturated esters this intermediate is in fact the E enolate as OMe has precedence over the O- for nomenclature
- Cannizzaro, C.E.; Houk, K.N. Magnitudes and Chemical Consequences of R3N+-C-H…O=C Hydrogen Bonding. J. Am. Chem. Soc. 2002, 124, 7163–7169. [Google Scholar] [CrossRef]
- Rafel, S.; Leahy, J.W. An Unexpected rate acceleration-practical improvements in the Baylis-Hillman Reaction. J. Org. Chem. 1997, 62, 1521–1522. [Google Scholar] [CrossRef]
- Robiette, R.; Aggarwal, V.K.; Harvey, J.N. Mechanism of the Morita−Baylis−Hillman reaction: A computational investigation. J. Am. Chem. Soc. 2007, 129, 15513–15525. [Google Scholar] [CrossRef]
- Kraftt, M.E.; Haxell, T.F.N.; Seibert, K.A.; Abboud, K.A. Implications in the Morita−Baylis−Hillman Alkylation: Isolation and Characterization of an Intermediate. J. Am. Chem. Soc. 2006, 128, 4174–4175. [Google Scholar]
- Reviews on catalytic enantioselective condensations: Palomo, C.; Oiarbide, M.; García, J.M. The Aldol Addition Reaction : An Old Transformation at Constant Rebirth. Chem. Eur. J. 2002, 8, 36–44. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P. The Direct Catalytic Asymmetric Cross-Aldol Reaction of Aldehydes. Angew. Chem. Int. Ed. 2003, 42, 858. [Google Scholar] [CrossRef]
- Heathcock, C.H. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Heathcock, C.H., Eds.; Pergamon: Oxford, UK, 1991; Volume 2, pp. 133–176. [Google Scholar]
- Teng, W.-D.; Huang, R.; Kwong, C.K.-W.; Shi, M.; Toy, P.H. Influence of Michael Acceptor Stereochemistry on Intramolecular Morita-Baylis-Hillman Reactions. J. Org. Chem. 2006, 71, 368–371. [Google Scholar]
- Heathcock, C.H. Asymmetric Syntheis; Morrison, J.D., Ed.; Academic Press, Inc.: New York, NY, USA, 1984; Volume 3, pp. 111–212. [Google Scholar]
- Trost, B.M; Fleming, I.; Heathcock, C.H. Comprehensive Organic Synthesis; Pergamon: Oxford, UK, 1991; Volume 2, pp. 173–179. [Google Scholar]
- Noyori, R.; Nishida, I.; Sakata, J. Erytro-selective aldol reaction via tris(dimethylamine)sulfonium enolates. J. Am. Chem. Soc. 1981, 103, 2106–2108. [Google Scholar] [CrossRef]
- Noyori, R.; Nishida, I.; Sakata, J.; Nishizawa, M. Tris(dialkylamine)sulfonium enolates. Synthesis, structure and reactions. J. Am. Chem. Soc. 1983, 105, 1598–1608. [Google Scholar] [CrossRef]
- Zimmerman, H.E.; Traxler, M.D. The Stereochemistry of the Ivanov and Reformatsky Reactions. J. Am. Chem. Soc. 1957, 79, 1920–1923. [Google Scholar] [CrossRef]
- According to nomenclature employed by Noyori’s group at the eighties syn aldols were named as erythro and anti aldols were named as threo
- Dubois, J-E.; Fort, J.-F. Dynamic stereochemistry of aldolization-XXI. Definition of “restoring energy” of a system of reversible competitive reactions. Tetrahedron 1972, 28, 1665–1675. [Google Scholar] [CrossRef]
- Murata, S.; Suzuki, M.; Noyori, R. Trialkylsilyl triflates. 5. A stereoselective aldol-type condensation of enol silyl ethers and acetals catalyzed by trimethylsilyl trifluoromethanesulfonate. J. Am. Chem. Soc. 1980, 102, 3248–3249. [Google Scholar] [CrossRef]
- Heathcock, C.H.; Davidsen, S.K.; Flippin, L.A. Acyclic stereoselection. 36. Simple diastereoselection in the Lewis acid mediated reactions of enol silanes with aldehydes. J. Org. Chem. 1986, 51, 3027–3037. [Google Scholar] [CrossRef]
- Denmark, S.E; Beutner, G.L.; Wynn, T.; Eastgate, M.D. Lewis base activation of lewis acids: catalytic, enantioselective addition of silyl ketene acetals to aldehydes. J. Am. Chem. Soc. 2005, 127, 3774–3789. [Google Scholar] [CrossRef]
- Denmark, S.; Chung, W.-j. Lewis Base activation of lewis acids: catalytic enantioselective glycolate aldol reactions. Angew. Chem. Int. Ed. 2008, 47, 1890–1892. [Google Scholar] [CrossRef]
- Abermil, M.; Masson, G.; Zhu, J. Highly Enantioselective aza Morita-Baylis-Hillman Reaction Catalyzed by Bifunctional β-isocupreidine Derivatives. J. Am. Chem. Soc. 2008, 130, 12596–12597. [Google Scholar]
- Abermil, M.; Masson, G.; Zhu, J. Invertible Enantioselectivity in 6’-Deoxy-6’-acylamino-β-Isocupreidine-catalyzed Aza-Morita-Baylis-Hillman Reaction: Key Role of an Achiral Additive. Org. Lett. 2009, 11, 4648–4651. [Google Scholar] [CrossRef]
- Ibawuchi, Y.; Nakatani, M.; Yokoyama, N.; Hatakeyama, S. Chiral amine-catalyzed asymmetric Baylis-Hillman reaction: A reliable route to highly enantiomerically-enriched to (α-Methylene-β-hydroxy)esters. J. Am. Chem. Soc. 1999, 121, 10219–10220. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.-F.; Niu, L.-F.; Xu, P.-X. Diastereo- and enantioselective aza-mbh-type reaction of nitroalkenes to n-tosylimines catalyzed by bifunctional organocatalysts. Org. Lett. 2009, 11, 3310–3313. [Google Scholar] [CrossRef]
- Dubois, J.E.; Dubois, M. The aldol condensation: influence of the solvent and the cation on stereoselectivity. Chem Commun. 1968, 1567–1568. [Google Scholar]
- Roy, D.; Sunoj, R.B. Ab Initio and Density Functional Theory Evidence on the Rate-Limiting Step in the Morita−Baylis−Hillman Reaction. Org. Lett. 2007, 9, 4873–4876. [Google Scholar] [CrossRef]
- Roy, D.; Sunoj, R.B. Water Catalysis in the Morita-Baylis-Hillman Reaction: A Mechanistic Perspective. Chem. Eur. J. 2008, 14, 10530–10534. [Google Scholar] [CrossRef]
- Fan, J.-F.; Yang, C.-H.; He, L.-J. DFT Study on the Role of Methanol Solvent in Morita-Baylis-Hillman Reaction. J. Int. Q. Chem. 2009, 109, 1311–1321, See also [62]. [Google Scholar] [CrossRef]
- Xu, J. Probing the mechanism of Morita-Baylis-Hillman reaction in dichloromethane by density functional theory. J. Mol. Struct. (TeoChem.) 2006, 767, 61–66, See also [62]. [Google Scholar] [CrossRef]
- Garnier, J.-M.; Anstiss, C.; Liu, F. Enantioselective Trifunctional Organocatalysis for Rate-Enhanced aza-Morita-Baylis-Hillman Reactions at Room Temperature. Adv. Synth. Catal. 2009, 351, 331–338. [Google Scholar] [CrossRef]
- Garnier, J.-M.; Liu, F. Trifunctional organocatalyst-promoted counterion catalysis for fast and enantioselective aza-Morita-Baylis-Hillman reactions at ambient temperature. Org. Biomol. Chem. 2009, 7, 1272–1275. [Google Scholar] [CrossRef]
- Jones, C.E.S.; Turega, S.M.; Clarke, M.L.; Philp, D. A rationally designed cocatalyst for the Morita−Baylis−Hillman reaction. Tetrahedron Lett. 2008, 49, 4666–4669. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, L.; Song, H.-L.; Hu, Y.; Wu, X.-Y. Chiral phosphinothiourea organocatalyst in the enantioselective Morita–Baylis–Hillman reactions of aromatic aldehydes with methyl vinyl ketone. Tetrahedron Lett. 2008, 49, 6262–6264. [Google Scholar]
- Wang, J.; Li, H.; Yu, X.; Zu, L.; Wang, W. Chiral binaphthyl-derived amine-thiourea organocatalyst-promoted asymmetric Morita-Baylis-Hillman reaction. Org. Lett. 2005, 7, 4293–4296. [Google Scholar] [CrossRef]
- Sohtome, Y.; Takemura, N.; Takagi, R.; Hashimoto, Y.; Nagasawa, K. Thiourea-catalyzed Morita–Baylis–Hillman reaction. Tetrahedron 2008, 64, 9423–9429. [Google Scholar] [CrossRef]
- McDougal, N. T.; Schaus, S. E. Asymmetric Morita-Baylis-Hillman Reactions Catalyzed by Chiral Brønsted Acids. J. Am. Chem. Soc. 2003, 125, 12094–12095. [Google Scholar] [CrossRef]
- Shi, M.; Liu, X.-G. Asymmetric Morita-Baylis-Hillman Reaction of Arylaldehydes with 2-Cyclohexen-1-one Catalyzed by Chiral Bis(Thio)urea and DABCO. Org. Lett. 2008, 10, 1043–1046. [Google Scholar] [CrossRef]
- Matsui, K.; Takizawa, S.; Sasai, H. Bifunctional organocatalysts for enantioselective aza-Morita-Baylis-Hillman reaction. J. Am. Chem. Soc. 2005, 127, 3680–3681. [Google Scholar] [CrossRef]
- Matsui, K.; Tanaka, K.; Horii, A.; Takizawa, S.; Sasai, H. Conformational lock in a Brønsted acid–Lewis base organocatalyst for the aza-Morita–Baylis–Hillman reaction. Tetrahedron: Asymmetry 2006, 17, 578–583. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.-H. Chiral phosphine Lewis base catalyzed asymmetric aza-Baylis–Hillman reaction of N-sulfonated imines with methyl vinyl ketone and phenyl acrylate. Chem. Commun. 2003, 1310–1311. [Google Scholar]
- Shi, M.; Chen, L.-H.; Teng, W.D. Asymmetric aza-Morita-Baylis-Hillman reaction of N-sulfonated imines with methyl vinyl ketone catalyzed by chiral phosphine Lewis bases bearing perfluoroalkanes as pony tails. Adv. Synth. Catal. 2005, 347, 1781–1789. [Google Scholar] [CrossRef]
- Shi, M.; Li, C.Q. Catalytic, asymmetric aza-Baylis–Hillman reaction of N-sulfonated imines with 2-cyclohexen-1-one and 2-cyclopenten-1-one in the presence of a chiral phosphine Lewis base. Tetrahedron Asymmetry 2005, 16, 1385–1391. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Chen, L.-C.; Shi, M. Asymmetric Aza-Morita–Baylis_Hillman Reaction of N-Sulfonated Imines with Activated Olefins Catalyzed by Chiral Phosphine Lewis Bases Bearing Multiple Phenol Groups. Adv. Synth. Catal. 2006, 348, 973–979. [Google Scholar] [CrossRef]
- Guan, X.- Y.; Jiang, Y.-Q.; Shi, M. Chiral Sterically Congested Phosphane-Amide Bifunctional Organocatalysts in Asymmetric Aza-Morita–Baylis–Hillman Reactions of N-Sulfonated Imines with Methyl and Ethyl Vinyl Ketones. Eur. J. Org. Chem. 2008, 2150–2155. [Google Scholar]
- Ito, K.; Nishida, K.; Gotanda, T. Highly enantioselective aza-Morita–Baylis–Hillman reaction with a bisphenol-based bifunctional organocatalyst. Tetrahedron Lett. 2007, 48, 6147–6149. [Google Scholar] [CrossRef]
- Richards, E.L.; Murphy, P.J.; Dinon, F.; Fratucello, S.; Brown, P.M.; Gelbrich, T.; Hurstouse, M.B. Assesing the scope of the tandem Michael/intramolecular aldol reaction mediated by secondary amines, thiols and phosphines. Tetrahedron 2001, 57, 7771–7784. [Google Scholar] [CrossRef]
- Dinon, F.; Richards, E.; Murphy, P.J.; Hibbs, D.E.; Hurtshouse, M.B.; Abdul Malik, K.M. Tandem intramolecular/aldol reactions mediated by secondary amines, thiols and phosphines. Tetrahedron Lett. 1999, 40, 3279–3282. [Google Scholar]
- Black, G.P.; Dinon, F.; Fratucello, S.; Murphy, P.J.; Nielsen, M.; Williams, H.L. Intramolecular Baylis-Hillman reaction catalyzed by secondary amines. Tetrahedron Lett. 1997, 38, 8561–8564. [Google Scholar]
- Shi, M.; Jiang, J.-K.; Li, C.-Q. Lewis base and L-proline co-catalyzed Baylis-Hillman reaction of arylaldehydes with metil vinyl ketone. Tetrahedron Lett. 2002, 43, 127–130. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, J.-K. An exploration of asymmetric Baylis-Hillman reactions catalyzed by quinidine-derived chiral amines. Tetrahedron Asymmetry 2002, 13, 1941–1947. [Google Scholar] [CrossRef]
- List, B.; Lerner, R.; Barbas, C.F., III. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Notz, W.; List, B. Catalytic Asymmetric Synthesis of anti-1,2-diols. J. Am. Chem. Soc. 2000, 122, 7386–7387. [Google Scholar] [CrossRef]
- Bui, T.; Barbas, III, C.F. A proline-catalyzed asymmetric Robinson annulation reaction. Tetrahedron Lett. 2000, 41, 6951–6954. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New strategies for organic catalysis: the First highly enantioselective organocatalytic Diels-Alder reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Houk, K.N.; Martin, H.J.; List, B. Quantum-mechanical predictions of the stereoselectivities of proline-catalyzed asymmetric intermolecular Aldol Additions. J. Am. Chem. Soc. 2003, 125, 2475–2479. [Google Scholar] [CrossRef]
- Tang, H.; Gao, P.; Zhao, G.; Zhou, Z.; He, L.; Tang, C. (1R,2R)-(-)-2-Dimethylamino-1-(4-nitrophenyl)-1,3-propanediol/L-proline cocatalyzed enantioselective Morita-Baylis-Hillman reaction. Cat. Commun. 2007, 8, 1811–1814. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, G.; Zhou, Z.; Gao, P.; He, L.; Tang, C. Chiral tertiary Amine/L-Proline Cocatalyzed Enantioselective Morita-Baylis-Hillman (MBH) Reaction. Eur. J. Org. Chem. 2008, 126–135. [Google Scholar]
- Imbriglio, J.E.; Vasbinder, M.M.; Miller, S.J. Dual catalyst control in the amino acid-peptide-catalyzed enantioselective Baylis-Hillman Reaction. Org. Lett. 2003, 5, 3741–3743. [Google Scholar] [CrossRef]
- Vasbinder, M.M.; Imbriglio, J.E.; Miller, S.J. Amino acid-peptide-catalyzed enantioselective Morita-Baylis-Hillman reactions. Tetrahedron 2006, 62, 11450–11459. [Google Scholar] [CrossRef]
- Aroyan, C.E.; Vasbinder, M.M.; Miller, S.J. Dual Catalyst Control in the Enantioselective Intramolecular Morita-Baylis-Hillman Reaction. Org. Lett. 2005, 7, 3849–3851. [Google Scholar] [CrossRef]
- Chen, S.-H.; Hong, B.-C.; Su, C.-F.; Sarshar, S. An unexpected inversion of enantioselectivity in the proline catalyzed intramolecular Baylis-Hillman reaction. Tetrahedron Lett. 2005, 46, 8899–8903. [Google Scholar] [CrossRef]
- Clemente, F.R.; Houk, K.N. Computational evidence for the enamine mechanism of intramolecular Aldol Reactions catalyzed by proline. Angew. Chem. Int. Ed. 2004, 43, 5766–5768. [Google Scholar] [CrossRef]
- Clemente, F.R.; Houk, K.N. Theoretical Studies of Stereoselectivities of Intramolecular Aldol Cyclizations Catalyzed by Amino Acids. J. Am. Chem. Soc. 2005, 127, 11294–11302. [Google Scholar] [CrossRef]
- Duarte, F.J.S.; Cabrita, E.J.; Frenking, G.; Gil Santos, A. Density Functional Study of Proline-Catalyzed Intramolecular Baylis-Hillman Reactions. Chem. Eur. J. 2009, 15, 1734–1746. [Google Scholar] [CrossRef]
- Pidathala, C.; Hoang, L.; Vignola, N.; List, B. Direct, Catalytic Asymmetric eno/exo Aldolizations. Angew. Chem. Int. Ed. 2003, 42, 2785–2788. [Google Scholar] [CrossRef]
- Utsumi, N.; Zhang, H.; Tanaka, F.; Barbas, III, C.F. A Way to highly enantiomerically enriched Aza-Morita-Baylis-Hillman-type products. Angew. Chem. Int. Ed. 2007, 46, 1878–1880. [Google Scholar]
- Vesely, J.; Dziedzic, P.; Córdova, A. Aza-Morita-Baylis-Hillman-type reactions: highly enantioselective organocatalytic addition of unmodified α,β-unsaturated aldehydes to N-BOC protected imines. Tetrahedron Lett. 2007, 48, 6900–6904. [Google Scholar] [CrossRef]
- List, B. the direct catalytic asymmetric three-component mannich reaction. J. Am. Chem. Soc. 2000, 126, 9336–9337. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Biller, W.T.; Martin, H.J. The proline-catalized asymmetric three component Mannich reaction: Scope, optimization, and applications to the highly enbantioselective synthesis of 1,2-aminoalcohols. J. Am. Chem. Soc. 2002, 124, 827–833. [Google Scholar]
- Gausepohl, R.; Buskens, P.; Kleinen, J.; Bruckman, A.; Lehmann, C.W.; Klankermayer, J.; Leitner, W. Highly enantioselective Aza-Baylis-Hillman in a chiral reaction medium. Angew. Chem. Int. Ed. 2006, 45, 3689–3692. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Mansilla, J.; Saá, J.M. Enantioselective, Organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman Reactions: Stereochemical Issues. Molecules 2010, 15, 709-734. https://doi.org/10.3390/molecules15020709
Mansilla J, Saá JM. Enantioselective, Organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman Reactions: Stereochemical Issues. Molecules. 2010; 15(2):709-734. https://doi.org/10.3390/molecules15020709
Chicago/Turabian StyleMansilla, Javier, and José M. Saá. 2010. "Enantioselective, Organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman Reactions: Stereochemical Issues" Molecules 15, no. 2: 709-734. https://doi.org/10.3390/molecules15020709



