Assessment of Antioxidant Capacity and Cytotoxicity of Selected Malaysian Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total phenolic content
2.2. Free radical scavenging activity
| Ethanolic extracts | Plant Part | Assays (1/IC50, mg/mL) | ||
|---|---|---|---|---|
| DPPH | Galvinoxyl | ABTS | ||
| Azadirachta indica | leaf | 1.35± 0.46 | 4.85 ± 0.08 | 6.01 ± 0.04 |
| Mangifera indica | leaf | 5.99 ± 0.02 | 19.86 ± 0.01 | 46.56 ± 0 |
| Garcinia mangostana | peel | 9.19 ± 0.02 | 37.56 ± 0.01 | 41.85 ± 0.02 |
| Nephelium lappaceum | peel | 8.56 ± 0.05 | 85.41 ± 0.01 | 62.99 ± 0 |
| Psidium guajava | leaf | 5.56 ± 0.08 | 30.88 ± 0.02 | 46.76 ± 0 |
| Fragaria x ananassa | leaf | 0.54 ± 0.80 | 2.53 ± 0.21 | 3.24 ± 0.11 |
| Lawsonia inermis | leaf | 0.79 ± 0.18 | 3.1 ± 0.23 | 8.83 ± 0.04 |
| Syzygium aqueum | leaf | 4.65 ± 0.02 | 11.98 ± 0.01 | 34.52 ± 0 |
| Nephelium lappaceum | leaf | 3.04 ± 0.03 | 71.04 ± 0.03 | 8.3 ± 0.07 |
| Peltophorum pterocarpum | leaf | 5.96 ± 0.12 | 34.76 ± 0.02 | 11.53 ± 0.08 |
| Peltophorum pterocarpum | bark | 9.90 ± 0.04 | 33.52 ± 0.01 | 9.11 ± 0.09 |
| Artocarpus champeden | leaf | 3.34 ± 0.21 | 11.54 ± 0.1 | 12.39 ± 0.04 |
| Nephelium mutobile | leaf | 4.10 ± 0.03 | 18.5 ± 0.04 | 10 ± 0.04 |
| Grape seed (commercial source) | seed | 3.75 ± 0.10 | 10.54 ± 0.03 | 28.18 ± 0.01 |
| Aqueous Extracts | Plant Part | Assays (1/IC50, mg/mL) | ||
|---|---|---|---|---|
| DPPH | Galvinoxyl | ABTS | ||
| Azadirachta indica | leaf | 1.05 ± 0.14 | 2.96 ± 0.1 | 2.41 ± 0.23 |
| Mangifera indica | leaf | 2.02 ± 0.39 | 4.65 ± 0.01 | 8.21 ± 0.03 |
| Garcinia mangostana | peel | 0.6 ± 2.36 | 1.39 ± 1.18 | 1.8 ± 0.76 |
| Nephelium lappaceum | peel | 1.86 ± 0.15 | 6.81 ± 0.06 | 5.46 ± 0.06 |
| Psidium guajava | leaf | 4.56 ± 0.01 | 7.5 ± 0.09 | 5.4 ± 0.03 |
| Fragaria x ananassa | leaf | 2.69 ± 0.07 | 3.56 ± 0.2 | 5.3 ± 0.11 |
| Lawsonia inermis | leaf | 0.27 ± 0.34 | 0.83 ± 0.32 | 1.08 ± 0.07 |
| Syzygium aqueum | leaf | 3.07 ± 0.07 | 6.68 ± 0.04 | 5.22 ± 0.07 |
| Nephelium lappaceum | leaf | 1.49 ± 0.02 | 3.64 ± 0.09 | 3.96 ± 0.06 |
| Peltophorum pterocarpum | leaf | 6.21 ± 0.05 | 30.85 ± 0.01 | 9.63 ± 0.02 |
| Peltophorum pterocarpum | bark | 5.05 ± 0.12 | 24.58 ± 0.01 | 9.02 ± 0.04 |
| Artocarpus champeden | leaf | 4.54 ± 0.01 | 14.33 ± 0.04 | 9.52 ± 0.03 |
| Nephelium mutobile | leaf | 0.27 ± 0.27 | 1.25 ± 0.09 | 0.9 ± 0.37 |
| Grape seed (commercial source) | seed | 2.18 ± 0.18 | 2.07 ± 0.33 | 5.19 ± 0.12 |
| Vitamin C | NA | 34.45 ± 0.04 | NA | NA |
| Emblica TM | NA | 3.36 ± 0.05 | NA | NA |
2.3. Inhibition of Lipid Peroxidation
2.4. Elemental Analysis
| Extracts | Plant Parts | Type of Tests (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lead (Pb) | Arsenic (As) | Mercury (Hg) | Calcium (Ca) | Iron (Fe) | Potassium (K) | Magnesium (Mg) | Sodium (Na) | ||
| Malaysian Standards* | NA | 10 | 5 | 0.5 | NA | NA | NA | NA | NA |
| European Standards** | NA | 3 | 1 | 0.1 | NA | NA | NA | NA | NA |
| Azadirachta indica | leaf | 0.52 ± 0.02 | <0.01 | 0.22 ± 0.02 | 29217 ± 536 | 12 ± 3 | 14883 ± 253 | 2332 ± 146 | <852 |
| Mangifera indica | leaf | < 0.15 | <0.01 | < 0.02 | 40937±423 | 50 ± 5 | 4975 ± 123 | 2693 ±125 | <905 |
| Garcinia mangostana | peel | 0.62 ± 0.03 | <0.01 | <0.02 | 1130 ± 53 | <1.5 | 7862 ± 65 | 495 ± 25 | <902 |
| Nephelium lappaceum | peel | 0.36 ± 0.02 | <0.01 | < 0.02 | 5073 ± 83 | <1.4 | 5510 ± 63 | 1402 ± 75 | <847 |
| Psidium guajava | leaf | 1.2 ± 0.1 | <0.01 | < 0.02 | 20038 ± 536 | 55 ± 5 | 6022 ± 763 | 2508 ± 128 | <875 |
| Fragaria x ananassa | leaf | 0.27 ± 0.02 | <0.01 | < 0.02 | 15540 ± 866 | <1.5 | 29805 ± 986 | 3532 ± 253 | <901 |
| Lawsonia inermis | leaf | 0.64 ± 0.03 | <0.01 | < 0.02 | 13276 ± 654 | 17 ± 3 | 6214 ± 532 | 3003 ± 531 | <887 |
| Syzygium aqueum | leaf | 0.21 ± 0.02 | <0.01 | < 0.02 | 9649 ± 423 | <1.5 | 8223 ± 332 | 2347 ± 75 | <908 |
| Nephelium lappaceum | leaf | <0.15 | <0.01 | < 0.02 | 27507 ± 1536 | 6.8 ± 0.1 | 6165 ± 56 | 2597 ± 23 | <891 |
| Peltophorum pterocarpum | leaf | <0.5 | 1.95 ± 0.1 | 0.02 ± 0.01 | 3383 ± 353 | 1602±21 | 7564 ± 86 | 3363 ± 47 | <913 |
| Peltophorum pterocarpum | bark | <0.5 | 3.86 ± 0.2 | <0.01 | 15110 ± 875 | 196 ± 10 | 1670 ± 63 | 1123 ± 46 | <857 |
| Artocarpus champeden | leaf | <0.5 | 0.4 ± 0.03 | <0.01 | 1580 ± 632 | <1.5 | 7601 ± 96 | 2521 ± 53 | <910 |
| Nephelium mutobile | leaf | <0.5 | 0.57 ± 0.03 | 0.03 ± 0.01 | 3748 ± 84 | <1.4 | 4722 ± 86 | 2390 ±63 | <866 |
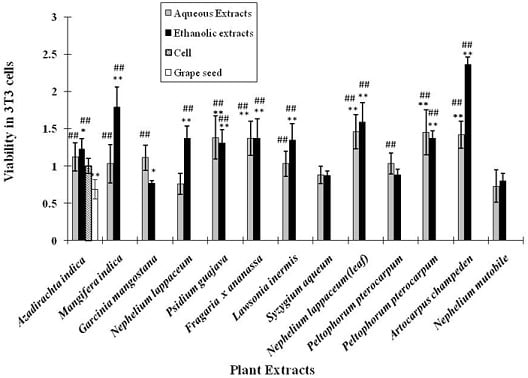
2.5. Cytotoxicity activity of the plant extracts
3. Experimental
General
4. Conclusions
Acknowledgements
References
- Huang, H.L.; Wang, B.G. Antioxidant capacity and lipophilic content of seaweeds collected from the qingdao coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Food Phenolics: Phenolics in cereal, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 4, 1523–1542. [Google Scholar]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables-the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Vinson, A.; Xuehui, S.; Ligia, Z.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Mukai, K.; Nagai, S.; Ohara, K. Kinetic study of the quenching reaction of singlet oxygen by tea catechins in ethanol solution. Free Radic. Biol. Med. 2005, 39, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Bosque, M.A.; Domingo, J.L.; Corbella, J. Dietary intake of lead and cadmium from foods in Tarragona Province, Spain. Bull. Environ. Contam. Toxicol. 1991, 46, 320–328. [Google Scholar] [CrossRef]
- WHO. Expert committee on specification for pharmaceuticals preparation; WHO Technical Report Series 823; WHO: Geneva, Switzerland, 1992; Volume 32, pp. 44–52, 75–76. [Google Scholar]
- Orech, F.O.; Akenga, T.; Ochora, J.; Friis, H.; Aagaard-Hansen, J. Potential toxicity of some traditional leafy vegeteables consumed in Nyang’Oma division, western kenya. Afr. J. Food Agric. Nutr. Dev. 2005, 5, 1–13. [Google Scholar]
- Jacopic, J.; Veberic, R.; Stampar, F. Extraction of phenolic compounds from green walnut fruits in different solvents. Acta Agric. Slov. 2009, 93, 11–15. [Google Scholar]
- Buenger, J.; Ackermann, H.; H-Jentzsch, A.; Mehling, A.; Pfitzner, I.; Reiffen, K.A.; Schroeder, K.R.; Wollenweber, U. An interlaboratory comparison of methods used to assess antioxidant potentials. Int. J. Cosmet. Sci. 2006, 28, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Bjork, L.; Trajkovski, V.; Uggla, M. Evaluation f antioxidant activities of rosehip ethanol extracts in different test systems. J. Sci. Food Agric. 2000, 80, 2021–2027. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jaganmohan, R.L. Phenolic constituents from the lichen parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z. Naturforsch., C, J. Biosci. 2000, 55, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Z.; Liu, Y.M.; Fratkins, J.Dm.; LeBlanc, M.H. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain Res. Bull. 2005, 66, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Food Act 1983 (ACT 281) & Regulations; International Law Book Services: Kuala Lumpur, Malaysia, 2004; p. 240.
- EC. Regulation No.1881/2006. In Setting Maximum Levels for Certain Contaminants in Food Stuffs; European Commission: London, UK, 2006. [Google Scholar]
- Melt, M.L.; Waugh, J.; Schneider, S.; Greene, N.D.; Rodriguez, C.; Hare, C. Mammalian cell cytotoxicity of diesel engine emission fractions. J. Appl. Toxicol. 2006, 1, 182–189. [Google Scholar] [CrossRef]
- Rucinska, A.; Roszczyk, M.; Gabryelak, T. Cytotoxicity of the isoflavone genistein in NIH 3T3 cells. Cell Biol. Int. 2008, 32, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Baral, R. Natural killer cell mediated cytotoxicity of tumor cells initiated by neem leaf preparation is associated with CD40–CD40L–mediated endogenous production of interleukin-12. Human Immunol. 2007, 68, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.T.; Yap, S.A.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Standardised Mangifera indica extract is an ideal antioxidant. Food Chem. 2009, 113, 1154–1159. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Beek, T.A.V. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2005, 85, 231–237. [Google Scholar] [CrossRef]
- EPA. Method 3052 Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, 3rd ed.; Environmental Protection Agency: Washington, DC, USA, 1996a. [Google Scholar]
- EPA. Method 6010B Inductively Coupled Plasma-Atomic Emission Spectrometry. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, 3rd ed.; Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- EPA. Method 245.6. In Determination of Mercury in Tissues by Cold Vapor Atomic Absorption Spectrometry, Revision 2.3.; Environmental Protection Agency: Washington, DC, USA, 1991. [Google Scholar]
- Palanisamy, U.; Cheng, H.M.; Masilamani, T.; Subramaniam, T.; Ling, L.T.; Radakrishnan, A.K. Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008, 109, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae a potent postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pinto, M.; Kwon, Y.I.; Apostolidis, E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Potential of Gingko biloba L. leaves in the management of hyperglycemia and hypertension using in vitro models. Bioresour. Technol. 2009, 100, 6599–6609. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are available from the authors. |


| Plant | Plant Part | Phenolic content (mg/g GA equivalent) | |
|---|---|---|---|
| Aqueous Extract | Ethanolic Extract | ||
| Azadirachta indica | leaf | 112 ± 13 | 139 ± 64 |
| Mangifera indica | leaf | 115 ± 14 | 648 ± 106 |
| Garcinia mangostana | peel | 135 ± 96 | 249 ± 135 |
| Nephelium lappaceum | peel | 212 ± 68 | 762 ± 60 |
| Psidium guajava | leaf | 211 ± 38 | 421 ± 114 |
| Fragaria x ananassa | leaf | 79 ± 15 | 86 ± 21 |
| Lawsonia inermis | leaf | 55 ± 17 | 135 ± 28 |
| Syzygium aqueum | leaf | 186 ± 44 | 524 ± 176 |
| Nephelium lappaceum | leaf | 194 ± 19 | 380 ± 40 |
| Peltophorum pterocarpum | leaf | 134 ± 22 | 475 ± 99 |
| Peltophorum pterocarpum | bark | 272 ± 117 | 1204 ± 602 |
| Artocarpus champeden | leaf | 279 ± 76 | 410 ± 128 |
| Nephelium mutobile | leaf | 53 ± 33 | 127 ± 3 |
| Grape seed (commercial source) | seed | 130 ± 7 | 605 ± 33 |
| Plants | Plant Part | Assay (1/IC50, mg/mL) | |
|---|---|---|---|
| Aqueous Extract | Ethanolic Extract | ||
| Azadirachta indica | leaf | 0.44 ± 2.38 | 1.4 ± 0.34 |
| Mangifera indica | leaf | 0.72 ± 0.95 | 3.11 ± 0.13 |
| Garcinia mangostana | peel | 3.88 ± 0.01 | 5.86 ± 0.05 |
| Nephelium lappaceum | peel | 1.44 ± 0.09 | 3.42 ± 0.1 |
| Psidium guajava | leaf | 2.56 ± 0.05 | 1.61 ± 0.18 |
| Fragaria x ananassa | leaf | 0.35 ± 1.86 | 0.32 ± 0.95 |
| Lawsonia inermis | leaf | 0.2 ± 0.2 | 0.3 ± 0.13 |
| Syzygium aqueum | leaf | 1.2 ± 0.09 | 2.4 ± 0.06 |
| Nephelium lappaceum | leaf | 0.69 ± 0.49 | 2.64 ± 0.1 |
| Peltophorum pterocarpum | leaf | 1.29 ± 0.15 | 2.74 ± 0.07 |
| Peltophorum pterocarpum | bark | 3.65 ± 0.1 | 5.68 ± 0.06 |
| Artocarpus champeden | leaf | 3.73 ± 0.05 | 2.08 ± 0.24 |
| Nephelium mutobile | leaf | 0.32 ± 1.19 | 3.35 ± 0.08 |
| Grape seed (commercial source) | seed | 1.99 ± 0.19 | 16.76 ± 0.02 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ling, L.T.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Assessment of Antioxidant Capacity and Cytotoxicity of Selected Malaysian Plants. Molecules 2010, 15, 2139-2151. https://doi.org/10.3390/molecules15042139
Ling LT, Radhakrishnan AK, Subramaniam T, Cheng HM, Palanisamy UD. Assessment of Antioxidant Capacity and Cytotoxicity of Selected Malaysian Plants. Molecules. 2010; 15(4):2139-2151. https://doi.org/10.3390/molecules15042139
Chicago/Turabian StyleLing, Lai Teng, Ammu Kutty Radhakrishnan, Thavamanithevi Subramaniam, Hwee Ming Cheng, and Uma D. Palanisamy. 2010. "Assessment of Antioxidant Capacity and Cytotoxicity of Selected Malaysian Plants" Molecules 15, no. 4: 2139-2151. https://doi.org/10.3390/molecules15042139