Characterization of the Active Constituents in Shixiao San Using Bioactivity Evaluation Followed by UPLC-QTOF and Markerlynx Analysis
Abstract
:1. Introduction
2. Results and Discussion
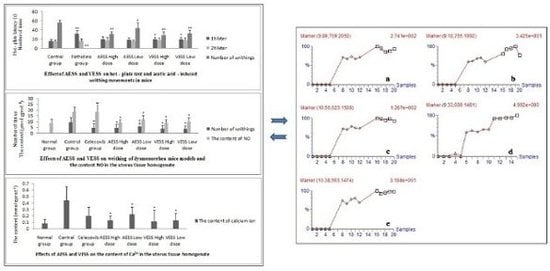
2.1. Effect of AESS and VESS on hot - plate test in mice
| Group | Dosage (g/kg) | Hot - plate latency (s) | ||
|---|---|---|---|---|
| 1 h | 2 h | |||
| Control group | - | 15.84 ± 3.81 | 15.40 ± 3.64 | |
| Pethidine group | 0.025 | 32.37 ± 6.56** | 16.45 ± 4.11 | |
| AESS group | High dose | 4.90 | 19.71 ± 5.90 | 17.48 ± 6.74 |
| Low dose | 2.45 | 19.40 ± 3.73 | 15.40 ± 3.66 | |
| VESS group | High dose | 10.06 | 20.37 ± 3.56* | 18.33 ± 4.53 |
| Low dose | 5.03 | 20.21 ± 3.78* | 18.13 ± 5.49 | |
2.2. Effect of AESS and VESS on acetic acid - induced writhing movements in mice
 ± s, n = 10)#.
± s, n = 10)#.
| Group | Dosage (g/kg) | Number of writhings | Inhibition (%) | |
|---|---|---|---|---|
| Control group | - | 56.10 ± 5.86 | - | |
| Pethidine group | 0.025 | 0.00 ** | 100.00 | |
| AESS group | High dose | 4.90 | 31.50 ± 5.19** | 43.85 |
| Low dose | 2.45 | 44.60 ± 12.06* | 20.50 | |
| VESS group | High dose | 10.06 | 29.11 ± 7.01** | 48.10 |
| Low dose | 5.03 | 32.70 ± 7.35** | 47.71 | |
2.3. Effects of AESS and VESS on dysmenorrhea mice models
| Group | Dose (g/kg) | Number of writhings | Inhibition (%) | |
|---|---|---|---|---|
| Normal group | - | 0 | 100 | |
| Control group | - | 9.75 ± 3.86 | - | |
| Celecoxib group | 0.0411 | 4.73 ± 3.87* | 51.52 | |
| AESS group | High dose | 4.90 | 5.00 ± 3.02* | 48.71 |
| Low dose | 2.45 | 5.92 ± 2.93* | 39.25 | |
| VESS group | High dose | 10.06 | 4.00 ± 2.66* | 58.97 |
| Low dose | 5.03 | 3.83 ± 2.66* | 60.68 | |
 ± s, n = 10)#.
± s, n = 10)#.
| Group | Dose (g/kg) | Ca2+/ mmol·gprot-1 | NO/ μmol·gprot-1 | |
|---|---|---|---|---|
| Normal group | - | 0.08 ± 0.07 | 9.12 ± 3.24 | |
| Control group | - | 0.44 ± 0.21 | 18.62 ± 4.12 | |
| Celecoxib group | 0.0411 | 0.20 ± 0.14 | 18.73 ± 7.25 | |
| AESS group | High dose | 4.90 | 0.13 ± 0.07* | 9.15 ± 2.34* |
| Low dose | 2.45 | 0.22 ± 0.12* | 11.98 ± 3.44* | |
| VESS group | High dose | 10.06 | 0.11 ± 0.18* | 9.02 ± 2.95* |
| Low dose | 5.03 | 0.13 ± 0.11* | 10.46 ± 3.05* | |
2.4. UPLC - QTOF - MS analytic results

| NO. | Retention time (min) | Identification | UV λ max (nm) | ESI+ | ESI- | Source herb |
|---|---|---|---|---|---|---|
| 1 | 6.79 | unknown | 225 | - | 231 [M+H]+, 253[M+Na]+ | FT |
| 2 | 8.83 | unknown | 228, 272 | - | 277 [M+H]+, 299 [M+Na]+ | PT/FT |
| 3 | 9.11 | quercetin-3-O-(2G-α-l-rhamnosyl)-rutinoside | 255, 353 | 755 [M−H]−, 300 [M−H−rha-glu-rha]−, 271 [M−2H−rha-glu-rha−CO]−, 255 [M−2H−rha-glu-rha−CO2]− | 757 [M+H]+ | PT |
| 4 | 9.41 | quercetin-3-O-neohesperidoside | 255, 355 | 609 [M−H]−, 300 [M−H−rha-glu]−, 271 [M−2H−rha-glu−CO]−, 255 [M−2H− rha-glu−CO2]− | 611 [M+H]+ | PT |
| 5 | 9.70 | kaempferol-3-O-(2G-α-l-rhamnosyl)-rutinoside | 265, 348 | 739 [M−H]−, 284 [M−H−rha-glu-rha]−, 255 [M−2H−rha-glu-rha−CO]− | 741 [M+H]+, 763[M+Na]+ | PT |
| 6 | 9.81 | isorhamnetin-3-O-(2G-α-l-rhamnosyl)-rutinoside | 254, 354 | 769 [M−H]−, 314 [M−H−rha-glu-rha]−, 285 [M−2H−rha-glu-rha−CO]− | 771 [M+H]+, 793[M+Na]+ | PT |
| 7 | 10.28 | kaempferol-3-O-neohesperidoside | 265, 348 | 593 [M−H]−, 284 [M−H−rha-glu-rha]−, 255 [M−2H−rha-glu-rha−CO]− | 595 [M+H]+, 617 [M+Na]+ | PT |
| 8 | 10.47 | isorhamnetin-3-O-neohesperidoside | 254, 354 | 623 [M−H]−, 314 [M−H−rha-glu-rha]−, 285 [M−2H−rha-glu-rha−CO]− | 625 [M+H]+, 647 [M+Na]+ | PT |
| 9 | 11.24 | isorhamnetin-3-O-rutinoside | 254, 354 | 623 [M−H]−, 314 [M−H−rha-glu-rha]−, 285 [M−2H−rha-glu-rha−CO]− | 625 [M+H]+, 647 [M+Na]+ | PT |
| 10 | 12.55 | unknow | - | - | 679 [M+H]+, 701 [M+Na]+ | PT/FT |
| 11 | 16.42 | unknow | - | - | 621 [M+H]+, 643 [M+Na]+ | FT |
| 12 | 16.87 | 2α,12β-dihydroxy-7,15–isopimardiene–18–oic-acid | - | 333 [M−H]− | 335 [M+H]+ | FT |
| 13 | 17.71 | unknow | - | - | 621 [M+H]+ | FT |
| 14 | 20.27 | 2α,15S,16-trihydroxy-8(14)–isopimarene–18–oic-acid | - | 351 [M−H]−, 387 [M+Cl]− | 353 [M+H]+ | FT |
| 15 | 36.87 | unknown | - | - | 274 [M+H]+ | FT |
| 16 | 11.79 | unknown | - | 513 [M−H]−, 365, 315 | - | FT |
| 17 | 12.71 | afzelin | 264 | 431 [M−H]− | 433 [M+H]+, 455 [M+Na]+ | FT |
| 18 | 16.05 | unknown | - | 349 [M−H]− | - | FT |
| 19 | 22.00 | unknown | - | 331 [M−H]−, 119 | - | FT |
| 20 | 24.93 | unknown | - | 413 [M−H]−, 331, 119 | - | FT |
| 21 | 26.07 | Quercetin-3,3′-dimethyl ether | 254, 352 | 329 [M−H]−, 301[M−dimethyl ether]− | - | PT |
| 22 | 26.87 | unknown | - | 329 [M−H]−, 215 | - | FT |
| 23 | 29.02 | unknown | - | 464 [M−H]−, 411, 329 | - | FT |
| 24 | 37.58 | unknown | - | 391 [M−H]−, 325, 243 | - | FT |
2.5. MarkerlynxTM analytic results


2.6. Discussion
3. Experimental
3.1. Materials
3.2. Chemicals and reagents
3.3. Animals
3.4. Instrumentation
3.5. Preparation of samples
3.5.1. Preparation of aqueous extract of Shixiao San (AESS)
3.5.2. Preparation of vinegary extract of Shixiao San (VESS)
3.6. Hot - plate test in mice
3.7. Acetic acid - induced writhing response in mice
3.8. Dysmenorrhea mice model preparation
3.9. Statistical analysis
3.10. UPLC – QTOF - MS analytical conditions
3.11. MarkerLynxTM
4. Conclusion
Acknowledgements
- Sample Availability: Samples of the compounds quercetin-3-O-(2G-α-L–rhamnosyl)-rutinoside, afzelin, kaempferol-3-O-glucoside, kaempferol-3-O-neohesperidoside, quercetin-3,3′-dimethyl ether, quercetin-3-O-neohesperidoside, 2,12-dihydroxy-7,15-isopimardiene-18-oic-acid and 2,15S,16-tri-hydroxy-8(14)-isopimarene-18-oic-acid are available from the authors.
References and Notes
- Huang, Y.J.; Wu, X.Y. Research progression and clinical application of Shixiao San. Chin. Trad. Plant Med. 1995, 17, 40–41. [Google Scholar]
- Zhou, W.; Su, S.L.; Duan, J.A.; Tao, W.W. The association analysis of shixiaosan’s traditional utility and modern research. Chin. Trad. Plant Med. 2009, 31, 1058–1061. [Google Scholar]
- Li, Q.; Sun, J.N.; Xia, X.H.; Xiao, H.B.; Song, C.Y.; Lin, Q.; Li, X.Z. A Pharmacodynamic study on shixiaosan and its extracts. J. Beijing Univ. TCM 1999, 22, 48–49. [Google Scholar]
- Zhang, P.; Xia, X.H.; Li, Q. The effects of Anti - thrombosis and thrombolysis of diferent solvent extract of shixiaosan. China Pharm. 2003, 12, 44–45. [Google Scholar]
- Li, Q.; Lu, Y.R.; Lu, X.Z. The study on progression of Faeces Trogopterori. Zhongguo Zhong Yao Za Zhi 1998, 23, 570–573. [Google Scholar]
- Zhang, Q.Y.; Mao, J.H.; Liu, P.; Huang, Q.H.; Lu, J.; Xie, Y.Y.; Weng, L.; Zhang, Y.; Chen, Q.; Chen, S.J.; Chen, Z. A systems biology understanding of the synergistic effects of arsenic sulfide and imatinib in BCR/ABL-associated leukemia. Proc. Nat. Acad. Sci. USA 2009, 106, 3378–3383. [Google Scholar]
- Li, S.L.; Song, J.Z.; Qiao, C.F.; Zhou, Y.; Xu, H.X. UPLC–PDA–TOFMS based chemical profiling approach to rapidly evaluate chemical consistency between traditional and dispensing granule decoctions of traditional medicine combinatorial formulae. J. Pharm. Biomed. Anal. 2010, 52, 468–478. [Google Scholar] [CrossRef]
- Normile, D. ASIAN MEDICINE: The New Face of Traditional Chinese Medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef]
- Su, S.L.; Guo, J.M.; Duan, J.A.; Wang, T.J.; Qian, D.W.; Shang, E.X.; Tang, Y.P. Ultra-performance liquid chromatography-tandem mass spectrometry analysis of the bioactive components and their metabolites of Shaofu Zhuyu decoction active extract in rat plasma. J. Chromatogr. B. 2010, 878, 355–362. [Google Scholar] [CrossRef]
- Salvemini, D.; Misko, T.P.; Masferrer, J.L.; Seibert, K.; Currie, M.G.; Needleman, P. Nitric oxide activates cyclooxygenase enzymes. Proc. Nat. Acad. Sci. USA 1993, 90, 7240–7244. [Google Scholar]
- Dawood, M.Y. Primary dysmenorrhea: advances in pathogenesis and management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Raine-Fenning, N. Dysmenorrhoea. Obstet. Gynaecol. Reprod. Med. 2008, 18, 294–299. [Google Scholar] [CrossRef]
- Li, T.; Roy, R. Studying traditional Chinese Medicine. Science 2003, 300, 740–741. [Google Scholar] [CrossRef]
- Su, S.L.; Hua, Y.Q.; Duan, J.A.; Shang, E.X.; Tang, Y.P.; Bao, X.J.; Lu, Y.; Ding, A.W. Hypothesis of active components in volatile oil from a Chinese herb formulation, Shao-Fu-Zhu-Yu decoction’, using GC-MS and chemometrics. J. Seperation Sci. 2008, 31, 1085–1091. [Google Scholar] [CrossRef]
- Qin, F.; Sun, H.X. Immunosuppressive activity of Pollen Typhae ethanol extract on the immune responses in mice. J. Ethnopharmacol. 2005, 102, 424–429. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, Z.Y. A study of the analgesic effect and acute toxicity of the extract of the Traditional Chinese Medicine Pollen Typhae. Her. Med. 2006, 25, 278–279. [Google Scholar]
- Park, W.H.; Kim, C.H.; Lee, Y.C.; Kim, C.H. Anti-inflammatory effects of a traditional Korean herbal formulation, Silsosangami, consisting of seven medicinal herbs: effect on hemolysis, neutrophil function, and gene expressions of iNOS and COX-2. Vasc. Pharmacol. 2004, 42, 7–15. [Google Scholar] [CrossRef]
- da Silva, B.P.; Bernardo, R.R.; Parente, J.P. Flavonol glycosides from Costus spicatus. Phytochemistry 2000, 53, 87–92. [Google Scholar]
- De Melo, G.O.; Malvar, D.D.; Vanderlinde, F.A.; Rocha, F.F.; Pires, P.A.; Costa, E.A.; de Matos, L.G.; Kaiser, C.R.; Costa, S.S. Antinociceptive and anti-inflammatory kaempferol kaempferol glycosides from Sedum dendroideum. J. Ethnopharmacol. 2009, 124, 228–232. [Google Scholar] [CrossRef]
- Adzu, B.; Amos, S.; Kapu, S.D.; Gamaniel, K.S. Anti - inflammatory and anti - nociceptive effects of Sphaeranthus senegalensis. J. Ethnopharmacol. 2003, 84, 169–173. [Google Scholar] [CrossRef]
- Fukawa, K.; Kawano, O.; Hibi, M.; Misaki, N.; Ohba, S.; Hatanaka, Y. A method for evaluating analgesic agents in rats. J. Pharmacol. Methods 1980, 4, 251–259. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar]
- Sun, H.Y.; Cao, Y.X.; Liu, J.; Gao, J.W.; Ma, M. The establishment of the dysmenorrhea model in mice. Chin. Pharmacol. Bull. 2002, 18, 233–235. [Google Scholar]
- Ostad, S.N.; Soodi, M.; Shariffzadeh, M.; Khorshidi, N.; Marzban, H. The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study. J. Ethnopharmacol. 2001, 76, 299–304. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, W.; Su, S.-L.; Duan, J.-A.; Guo, J.-M.; Qian, D.-W.; Shang, E.-X.; Zhang, J. Characterization of the Active Constituents in Shixiao San Using Bioactivity Evaluation Followed by UPLC-QTOF and Markerlynx Analysis. Molecules 2010, 15, 6217-6230. https://doi.org/10.3390/molecules15096217
Zhou W, Su S-L, Duan J-A, Guo J-M, Qian D-W, Shang E-X, Zhang J. Characterization of the Active Constituents in Shixiao San Using Bioactivity Evaluation Followed by UPLC-QTOF and Markerlynx Analysis. Molecules. 2010; 15(9):6217-6230. https://doi.org/10.3390/molecules15096217
Chicago/Turabian StyleZhou, Wei, Shu-Lan Su, Jin-Ao Duan, Jian-Ming Guo, Da-Wei Qian, Er-Xin Shang, and Jin Zhang. 2010. "Characterization of the Active Constituents in Shixiao San Using Bioactivity Evaluation Followed by UPLC-QTOF and Markerlynx Analysis" Molecules 15, no. 9: 6217-6230. https://doi.org/10.3390/molecules15096217