Antitrypanosomal Activity of Novel Benzaldehyde-Thiosemicarbazone Derivatives from Kaurenoic Acid †
Abstract
:1. Introduction
2. Results and Discussion
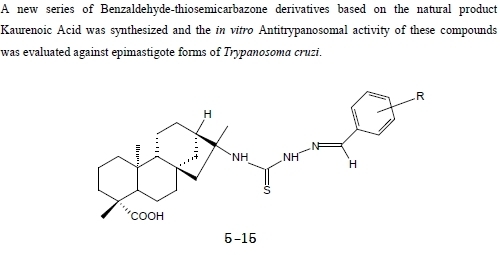
2.1. Synthesis

2.2. Biological assays
| LLMCK2 CC50 | Epimastigote IC50 | ||
|---|---|---|---|
| μM ± SDb | SIa | ||
| 1 | 59.5 ± 0.1 | 101.7 ± 0.0 | 0.6 |
| 2 | 52.0 ± 0.3 | 58.2 ± 0.2 | 0.9 |
| 3 | 103.4 ± 0.7 | 43.4 ± 5.6 | 2.4 |
| 4 | 71.4 ± 0.7 | 107.0 ± 13.0 | 0.7 |
| 5 | 31.1 ± 1.0 | 68.2 ± 11.2 | 0.5 |
| 6 | 122.4± 1.0 | 23.4 ± 7.7 | 7.3 |
| 7 | 78.2 ± 0.0 | 16.0 ± 4.4 | 5.7 |
| 8 | 29.5 ± 0.8 | 18.3 ± 4.0 | 1.9 |
| 9 | 248.5 ± 0.6 | 79.5 ± 17.9 | 3.2 |
| 10 | 17.1 ± 0.1 | 2.0 ± 1.1 | 9.0 |
| 11 | 104.8 ± 0.4 | 19.0 ± 0.0 | 5.6 |
| 12 | 15.2 ± 0.0 | 116.6 ± 12.8 | 0.1 |
| 13 | 364.2 ± 11.3 | 39.4 ± 14.3 | 8.4 |
| 14 | 56.2 ± 0.4 | 23.5 ± 7.0 | 3.2 |
| 15 | 28.7 ± 1.1 | 14.9 ± 0.3 | 2.0 |
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation of kaurenoic acid (1)
3.4. General procedure for the preparation of isothiocyanate 2
3.5. General procedure for the preparation of thiosemicarbazide 4
3.6. General procedure for the synthesis of benzaldehyde-thiosemicarbazones 5-15
3.7. Antitrypanosomal assay
3.8. Cytotoxicity assay
4. Conclusions
Acknowledgements
References and Notes
- de Souza, W. Basic cell biology of Trypanosoma cruzi. Curr. Pharm. 2002, 8, 269–285. [Google Scholar] [CrossRef]
- Teixeira, A.R.L.; Nitz, N.; Guimaro, M.C.; Gomes, C.; Santos-Buch, C.A. Chagas disease. Postgrad. Med. J. 2006, 82, 788–798. [Google Scholar] [CrossRef]
- Nwaka, S.; Ridley, R.G. Virtual drug discovery and development for neglected diseases through public–private partnerships. Nat. Rev. Drug Discov. 2003, 2, 919–928. [Google Scholar] [CrossRef]
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic Side Effects of Drugs Used to Treat Chaga’s Disease (American Trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef]
- Schofield, C.J.; Jannin, J.; Salvatella, R. The future of Chagas disease control. Trends Parasitol. 2006, 22, 583–588. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriès, M.A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. The biological activity of naturally occuring kaurane diterpenes. Fitoterapia 1997, 68, 303–325. [Google Scholar]
- García, P.A.; de Oliveira, A.B.; Batista, R. Occurrence, biological activities and synthesis of kaurane diterpenes and their glycosides. Molecules 2007, 12, 455–483. [Google Scholar] [CrossRef]
- Paiva, L.A.F.; Gurgel, L.A.; Silva, R.M.; Tomé, A.R.; Gramosa, N.V.; Silveira, E.R.; Santos, F.A.; Rao, V.S.N. Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffii on acetic acid-induced colitis in rats. Vasc. Pharmacol. 2003, 39, 303–307. [Google Scholar]
- Costa-Lotufo, L.V.; Cunha, G.M.A.; Farias, P.A.M.; Viana, G.S.B.; Cunha, K.M.A.; Pessoa, C.; Morais, M.O.; Silveira, E.R.; Gramosa, N.V.; Rao, V.S.N. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleoresin. Toxicon 2002, 40, 1231–1234. [Google Scholar] [CrossRef]
- Batista, R.; Humberto, J.L.; Chiari, E.; de Oliveira, A.B. Synthesis and trypanocidal activity of ent-kaurane glycosides. Bioorg. Med. Chem. 2007, 15, 381–391. [Google Scholar] [CrossRef]
- Saúde-Guimarães, D.; Faria, A.R. Substâncias da natureza com atividade anti-Trypanosoma cruzi. Rev. Bras. Farmacong. 2007, 172, 455–465. [Google Scholar] [CrossRef]
- Vieira, H.S.; Takahashi, J.A.; de Oliveira, A.B.; Chiari, E.; Boaventura, M.A. Novel derivatives of kaurenoic acid: preparation and evaluation of their trypanocidal activity. J. Braz. Chem. Soc. 2002, 13, 151–157. [Google Scholar] [CrossRef]
- Henry, G.E.; Adams, L.S.; Rosales, J.C.; Jacobs, H.; Heber, D.; Seeram, N.P. Kaurene diterpenes from Laetia thamnia inhibit the growth of human cancer cells in vitro. Cancer Lett. 2006, 244, 190–194. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgievi, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Boeck, P.; Sá, M.M.; Souza, B.S.; Cercená, R.; Escalante, A.M.; Zachino, S.A.; Cechinel-Filho, V.; Yunes, R.A. A simple synthesis of kaurenoic esters and other derivatives and evaluation of their antifungal activity. J. Braz. Chem. Soc. 2005, 16, 1360–1366. [Google Scholar] [CrossRef]
- Bresciani, L.F.V.; Cechinel-Filho, V.; Yunes, R.A. Comparative study of different parts of Wedelia paludosa by gas chromatography. Nat. Prod. Lett. 2000, 14, 247–254. [Google Scholar] [CrossRef]
- Medina, J.M.; Peixoto, J.L.B.; Silva, A.A.; Haraguchi, S.K.; Falavigna, D.L.M.; Zamuner, M.L.M.; Sarragiotto, M.H.; Vidotti, G.J. Evaluation of the molluscicidal and Schistosoma mansoni cercariae activity of Croton floribundus extracts and kaurenoic acid. Rev. Bras. Farmacong. 2009, 19, 207–211. [Google Scholar]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Tenório, R.P.; Carvalho, C.S.; Pessanha, C.S.; de Lima, J.G.; de Faria, A.R.; Alves, A.J.; de Melo, E.J.T.; Góes, A.J.S. Synthesis of thiosemicarbazone and 4-thiazolidinone derivatives and their in vitro anti-Toxoplasma gondii activity. Bioorg. Med. Chem. Lett. 2005, 15, 2575–2578. [Google Scholar] [CrossRef]
- Beraldo, H. Semicarbazones and thiosemicarbazones: their wide pharmacological profile and clinical applications. Quim. Nova 2004, 27, 461–471. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Pansare, S.V.; das Sarma, K.; Keith, K.A.; Kern, E.R. Combinatorial optimization of isatin-β-thiosemicarbazones as anti-poxvirus agents. J. Med. Chem. 2005, 48, 3045–3050. [Google Scholar] [CrossRef]
- Hu, W.-X.; Zhou, W.; Xia, C.-N.; Wen, X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2006, 16, 2213–2218. [Google Scholar] [CrossRef]
- Kolocouris, A.; Dimas, K.; Pannecuoque, C.; Witvrouw, M.; Foscolos, G.B.; Stamatiou, G.; Fytas, G.; Zoidis, G.; Kolocouris, N.; Andrei, G.; Snoeck, R.; de Clercq, E. New 2-(1-adamantylcarbonyl) pyridine and 1-acetyladamantane thiosemicarbazones–thiocarbonohydrazones: cell growth inhibitory, antiviral and antimicrobial activity evaluation. Bioorg. Med. Chem. Lett. 2002, 12, 723–727. [Google Scholar] [CrossRef]
- Tarasconi, P.; Copacchi, S.; Pelosi, G.; Cornia, M.; Albertini, R.; Bonati, A.; Dall’Aglio, P.P.; Lunghi, P.; Pinelli, S. Synthesis, spectroscopic characterization and biological properties of new natural aldehydes thiosemicarbazones. Bioorg. Med. Chem. 2000, 88, 157. [Google Scholar]
- de Oliveira, R.B.; de Souza-Fagundes, E.M.; Soares, R.P.P.; Andrade, A.A.; Kretti, A.U.; Zani, C.L. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 183–188. [Google Scholar]
- Pérez-Rebolledo, A.; Teixeira, L.R.; Batista, A.A.; Mangrich, A.S.; Aguirre, G.; Cerceretto, H.; González, M.; Hernández, P.; Ferreira, A.M.; Speziali, N.L.; Beraldo, H. 4-Nitroacetophenone-derived thiosemicarbazones and their copper (II) complexes with significant in vitro anti-trypanosomal activity. Eur. J. Med. Chem. 2008, 43, 939–948. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; Faundez, M. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef]
- Du, X.; Guoi, C.; Hansell, E.; Doyle, P.S.; Caffrey, C.R.; Holler, T.P.; James, H.; McKerrow, J.H.; Cohen, E. Synthesis and structure-activity relationship study of potent trypanocidal thio semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J. Med. Chem. 2002, 45, 2695–2707. [Google Scholar] [CrossRef]
- Fujii, N.; Mallari, J.P.; Hansell, E.; Mackey, Z.; Doyle, P.; Zhou, Y.M.; Gut, J.; Rosenthal, P.J.; McKerrow, J.H.; Guy, R.K. Discovery of potent thiosemicarbazone inhibitors of rhodesain and cruzain. Bioorg. Med. Chem. Lett. 2005, 15, 121–123. [Google Scholar] [CrossRef]
- Tenório, R.P.; Góes, A.J.S.; de Lima, J.G.; de Faria, A.R.; Alves, A.J.; Aquino, T.M. Thiosemicarbazones: preparation methods, synthetic applications and biological importance. Quim. Nova 2005, 28, 1030–1037. [Google Scholar] [CrossRef]
- da Silva, A.P.; Martini, M.V.; de Oliveira, C.M.A.; Cunha, S.; de Carvalho, J.E.; Ruiz, A.L.T.G.; da Silva, C.C. Antitumor activity of (-)-α-bisabolol-based thiosemicarbazones against human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar]
- Yamaguchi, M.U.; da Silva, A.P.B.; Ueda-Nakamura, T.; Dias-Filho, B.P.; da Silva, C.C.; Nakamura, C.V. Effects of a thiosemicarbazide camphene derivative on Trichophyton mentagrophytes. Molecules 2009, 14, 1796–1807. [Google Scholar] [CrossRef]
- da Silva, C.C.; Almagro, V.; Marsaioli, A.J. A direct route to terpene isothiocyanates. Tetrahedron Lett. 1993, 34, 6717–6720. [Google Scholar] [CrossRef]
- de Oliveira, C.M.A.; da Silva, C.C.; Collins, C.H.; Marsaioli, A.J. Controlling factors determining the selective HSCN addition to double bonds and their application to the synthesis of 7-isothiocyano-7,8-α-dihydro-bisabolene. J. Braz. Chem. Soc. 2001, 12, 661–666. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Haraguchi, S.K.; Silva, A.A.; Vidotti, G.J.; Dos Santos, P.V.; Garcia, F.P.; Pedroso, R.B.; Nakamura, C.V.; De Oliveira, C.M.A.; Da Silva, C.C. Antitrypanosomal Activity of Novel Benzaldehyde-Thiosemicarbazone Derivatives from Kaurenoic Acid †. Molecules 2011, 16, 1166-1180. https://doi.org/10.3390/molecules16021166
Haraguchi SK, Silva AA, Vidotti GJ, Dos Santos PV, Garcia FP, Pedroso RB, Nakamura CV, De Oliveira CMA, Da Silva CC. Antitrypanosomal Activity of Novel Benzaldehyde-Thiosemicarbazone Derivatives from Kaurenoic Acid †. Molecules. 2011; 16(2):1166-1180. https://doi.org/10.3390/molecules16021166
Chicago/Turabian StyleHaraguchi, Shirani K., Adriano A. Silva, Gentil J. Vidotti, Phercyles V. Dos Santos, Francielle P. Garcia, Raissa B. Pedroso, Celso V. Nakamura, Cecília M. A. De Oliveira, and Cleuza C. Da Silva. 2011. "Antitrypanosomal Activity of Novel Benzaldehyde-Thiosemicarbazone Derivatives from Kaurenoic Acid †" Molecules 16, no. 2: 1166-1180. https://doi.org/10.3390/molecules16021166