Investigation of the Interactions between the Hydrophobic Cavities of Cyclodextrins and Pullulanase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inclusion Hypothesis between β-CD and Pullulanase Molecules
2.2. Effect of Sizes of Hydrophobic Cavities on Pullulanase Activity



2.3. Effect of Side Chain Groups of β-CD on the Interaction between Cavities and Pullulanase Molecules
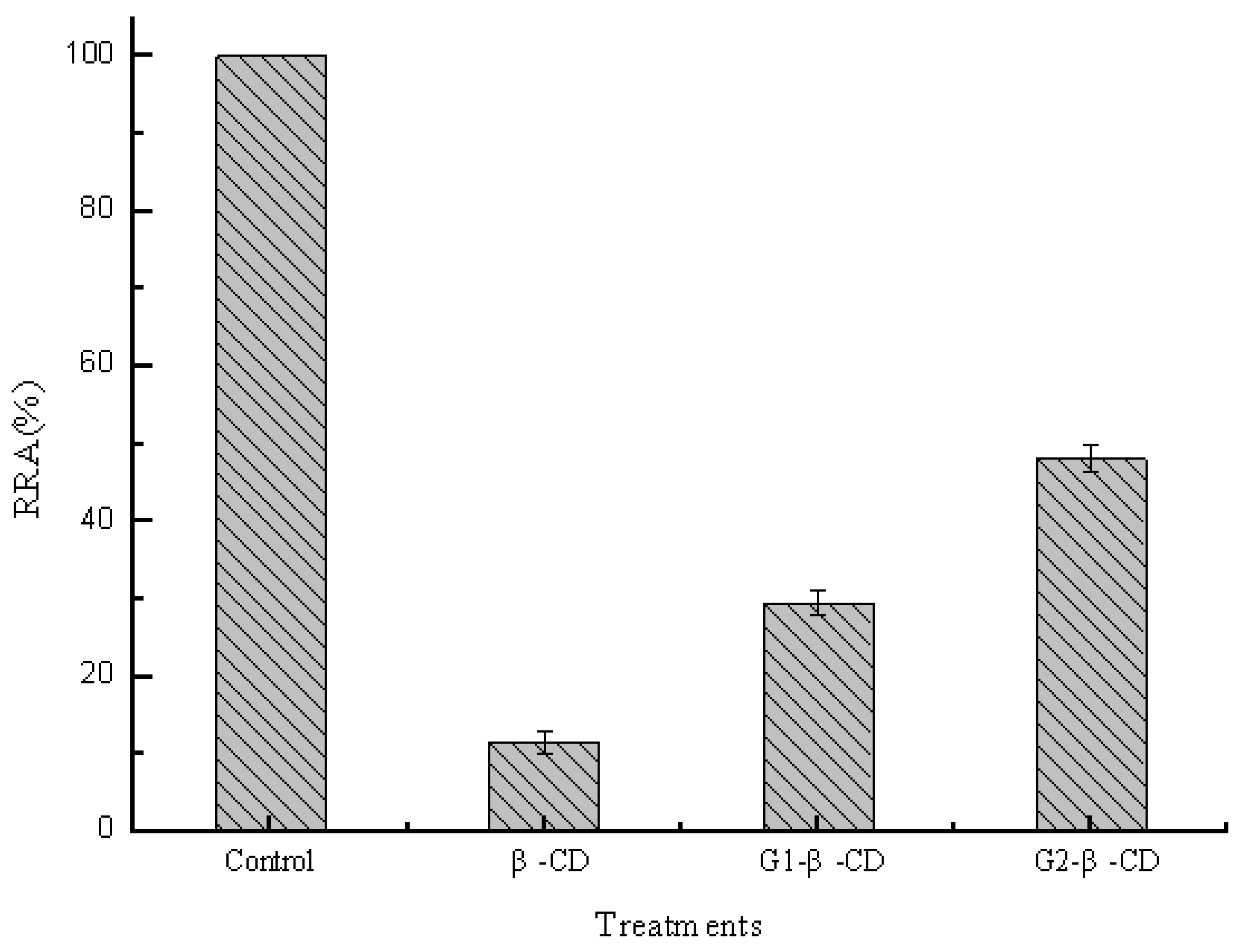
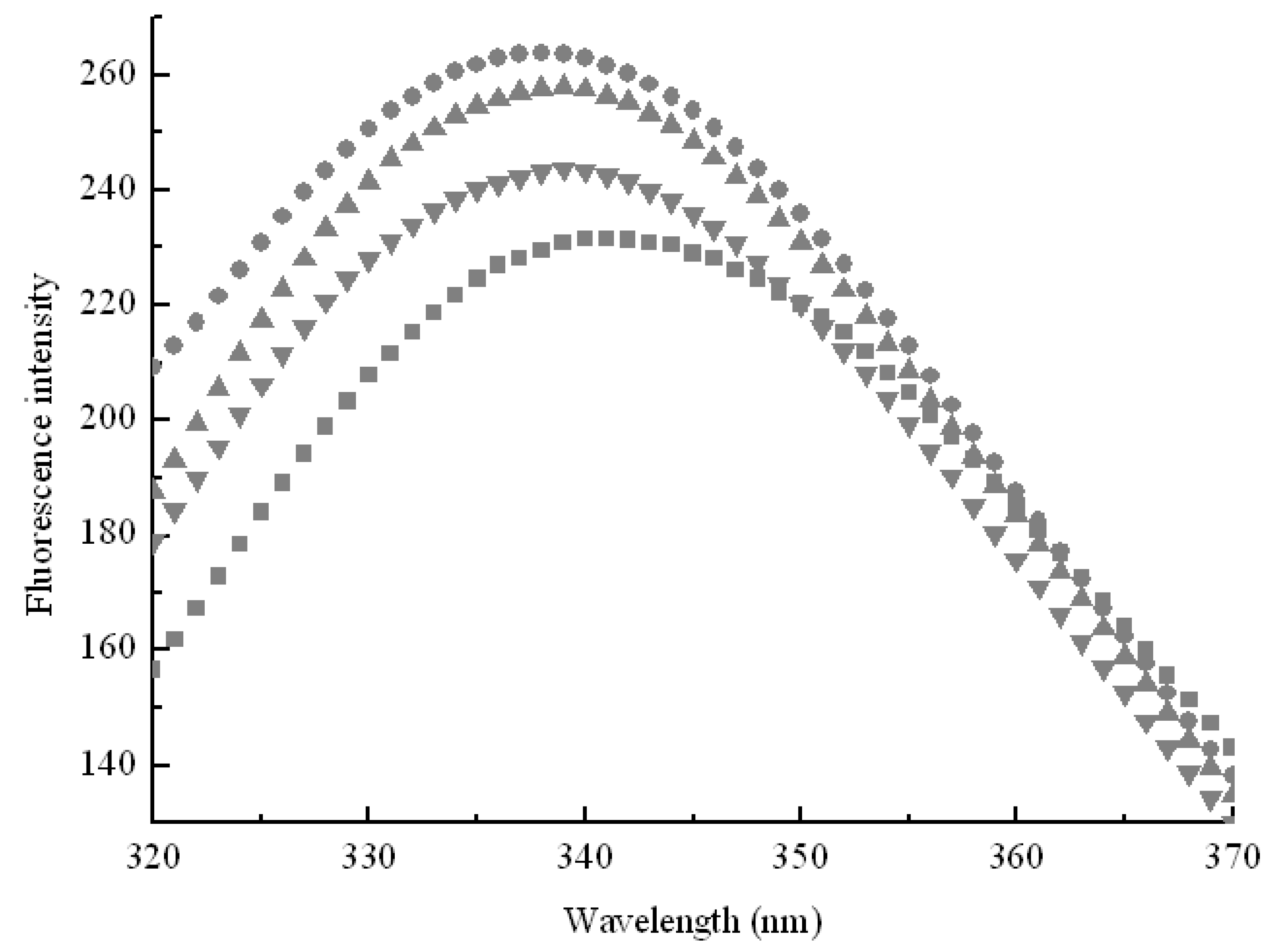
3. Experimental
3.1. Materials and Chemicals
3.2. Purification of Pullulanase from Bacillus Acidopullulyticus
3.3. Pullulanase Activity Assays
3.4. Fluorescence Spectroscopy
4. Conclusions
Acknowledgments
References
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1752. [Google Scholar] [CrossRef]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Hamilton, L.M.; Kelly, C.T.; Fogarty, W.M. Review: Cyclodextrins and their interaction with amylolytic enzymes. Enzyme Microb. Technol. 2000, 26, 561–567. [Google Scholar]
- Koralewska, A.; Augustyniak, W.; Temeriusz, A.; KaŃska, M. Effects of cyclodextrin derivatives on the catalytic activity of tyrosinephenol-Lyase. J. Incl. Phenom. Macrocycl. Chem. 2004, 49, 193–197. [Google Scholar] [CrossRef]
- Mcgarraghy, M.; Darcy, R. Effect of cyclodextrins on chymotrypsin action. J. Incl. Phenom. Macrocycl. Chem. 2000, 37, 259–264. [Google Scholar] [CrossRef]
- Pinotsis, N.; Leonidas, D.D.; Chrysina, E.D.; Oikonomakos, N.G.; Mavridis, I. The binding of β- and γ-cyclodextrins to glycogen phosphorylase b: kinetic and crystallographic studies. Protein Sci. 2003, 12, 1914–1924. [Google Scholar]
- Thoma, J.A.; Koshland, D.E. Competitive inhibition by substrate during enzyme action. Evidence for the induced-fit theory. J. Am. Chem. Soc. 1960, 82, 3329–3333. [Google Scholar]
- Vretblad, P. Immobilization of ligands for biospecific affinity chromatography via their hydroxyl groups. The cyclohexaamylose-bamylase system. FEBS Lett. 1974, 47, 86–89. [Google Scholar] [CrossRef]
- Lee, K.C.P.; Tao, B.Y. A kinetic study of cyclodextrin glycosyltransferase: Substrate and product inhibitions. Biotech. Appl. Biochem. 1995, 21, 111–121. [Google Scholar]
- Penninga, D.; Van Der Veen, B.A.; Knegtel, R.M.A.; Van Hijum, S.A.F.T.; Rozeboom, H.J.; Kalk, K.H.; Dijkstra, B.W.; Dijkhuizen, L. The raw starch binding domain of cyclodextrin glycosyltransferase from Bacillus circulans strain 251. J. Biol. Chem. 1996, 271, 32777–32784. [Google Scholar]
- Fagerstrom, R. Evidence for a polysaccharide-binding domain in Hormoconis resinae glucoamylase P: effects of its proteolytic removal on substrate specificity and inhibition by β-cyclodextrin. Microbiology 1994, 140, 2399–2407. [Google Scholar]
- Monma, M.; Yamamoto, Y.; Kainuma, K. Subsite structure of Chalara paradoxa glucoamylase and interaction of the glucoamylase with cyclodextrins. Agric. Biol. Chem. 1989, 53, 1503–1508. [Google Scholar] [CrossRef]
- Iwamoto, H.; Ohmori, M.; Ohno, M.; Hirose, J.; Tanaka, A.; Sakai, S.; Hiromi, K. Comparison of the binding of β-CD with pullulanase from Klebsiella pneumoniae as studied by equilibrium and kinetic fluorometry. J. Biochem. 1994, 116, 1264–1268. [Google Scholar]
- Marshall, J.J. Inhibition of pullulanase by Schardinger dextrins. FEBS Lett. 1973, 37, 269–273. [Google Scholar] [CrossRef]
- Yu, B.; Tian, Y.; Yang, N.; Xu, X.; Jin, Z. A study on the inhibition mechanism of β-cyclodextrin on pullulanase. J. Incl. Phenom. Macrocycl. Chem. 2010. [Google Scholar] [CrossRef]
- Siimer, E.; Kurvits, M.; Kostner, A. Thermochemical investigation of β-cyclodextrin complexes with benzoic acid and sodium benzoate. Thermochim. Acta 1987, 116, 249–256. [Google Scholar]
- Jonasson, P.; Aronsson, G.; Carlsson, U.; Jonsson, B. Tertiary structure formation at specific tryptophan side chains in the refolding of human carbonic anhydrase II. Biochemistry 1997, 36, 5142–5148. [Google Scholar]
- Miller, G.L. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, B.; Wang, J.; Zhang, H.; Jin, Z. Investigation of the Interactions between the Hydrophobic Cavities of Cyclodextrins and Pullulanase. Molecules 2011, 16, 3010-3017. https://doi.org/10.3390/molecules16043010
Yu B, Wang J, Zhang H, Jin Z. Investigation of the Interactions between the Hydrophobic Cavities of Cyclodextrins and Pullulanase. Molecules. 2011; 16(4):3010-3017. https://doi.org/10.3390/molecules16043010
Chicago/Turabian StyleYu, Bo, Jinpeng Wang, Huanxin Zhang, and Zhengyu Jin. 2011. "Investigation of the Interactions between the Hydrophobic Cavities of Cyclodextrins and Pullulanase" Molecules 16, no. 4: 3010-3017. https://doi.org/10.3390/molecules16043010



