The Effect of Alkali and Ce(III) Ions on the Response Properties of Benzoxazine Supramolecules Prepared via Molecular Assembly
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Monomers by the Mannich Reaction




2.2. Liquid Extraction of Alkali Metal Ions

| Benzoxazine derivatives | λmax (nm) | M+-picrates | λmax (nm) | M+-benzoxazine derivative complexes, λmax (nm) | ||
|---|---|---|---|---|---|---|
| Na+ | K+ | Cs+ | ||||
| BM1 | 288 | Na+-picrate | 355 | 376 | 432 | 422 |
| BM2 | 288 | K+-picrate | 355 | 378 | 435 | 430 |
| BM3 | 298 | Cs+-picrate | 355 | 396 | 450 | 445 |


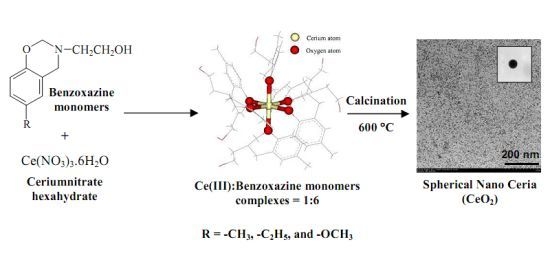
2.3. Complexation of Benzoxazine Monomers and Ce(III) Ion



2.4. Computational Simulation



| Ce(III)-benzoxazine monomer complexes | Ce(III)-BM1 | Ce(III)-BM2 | Ce(III)-BM3 |
|---|---|---|---|
| Crystallite size (nm) | 16 | 11 | 14 |

3. Experimental
3.1. Chemicals
3.2. Instruments
3.3. Preparation of Monomers by the Mannich Reaction
3.4. Liquid Extraction of Alkali Metal Ions
3.5. Complexation of Benzoxazine Monomers and Ce(III) Ion
3.6. Computational Simulation
3.7. Preparation of Ceria (CeO2) from Ce(III)-Benzoxazine Monomer Complexes
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds BM are available from the authors.
References and Notes
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2000; p. 116. [Google Scholar]
- Arduni, A.; Pochini, A.; Reverberi, S.; Ungaro, R. The preparation and properties of a new lipophilic sodium selective ether ester ligand derived from p-t-butylcalix[4]arene. Tetrahedron 1986, 42, 2089–2100. [Google Scholar] [CrossRef]
- Böhmer, V. Calixarenes, macrocycles with (almost) unlimited possibilities. Angrew. Chem. Int. Ed. Engl. 1995, 34, 713–745. [Google Scholar] [CrossRef]
- Sone, T.; Ohba, Y.; Yamazaki, H. Inclusion Properties of Acyclic p-Substituted Phenol-Formaldehyde Oligomers. Bull. Chem. Soc. Jpn. 1989, 62, 1111. [Google Scholar] [CrossRef]
- Yamagishi, T.; Tani, K.; Ishida, S.; Nakamoto, Y. Linear all-ortho oligomers of phenol-formaldehyde resins. Polym. Bull. 1994, 33, 281. [Google Scholar] [CrossRef]
- Chirachanchai, S.; Laobuthee, A.; Phongtamrug, S.; Siripattanasarakit, W.; Ishida, H. A novel ion extraction material using host-guest properties of oligobenzoxazine local structure and benzoxazine monomer molecular assembly. J. Appl. Polym. Sci. 2000, 77, 2561. [Google Scholar] [CrossRef]
- Laobuthee, A.; Chirachanchai, S.; Ishida, H.; Tashiro, K. Asymmetric Mono-oxazine: An Inevitable Product from Mannich Reaction of Benzoxazine Dimers. J. Am. Chem. Soc. 2001, 123, 9947. [Google Scholar]
- Laobuthee, A.; Chirachanchai, S. A simple, effective, and selective synthesis route for difunctional 30-membered macrocyclic ester and linear oligoester derived from benzoxazine dimer. Chem. Lett. 2002, 6, 613–614. [Google Scholar]
- Chirachanchai, S.; Phongtamrug, S.; Laobuthee, A. A simple, effective, and selective synthesis route without template effect (part II) for [2 + 2] difunctional 28-membered macrocyclic ethers based on benzoxazine dimers and its inclusion phenomena with metal ion. Chem. Lett. 2003, 5, 432–433. [Google Scholar]
- Laobuthee, A.; Ishida, H.; Chirachanchai, S. Metal ion guest responsive benzoxazine dimers and inclusion phenomena of cyclic derivatives. J. Incl. Phenom. Macrocycl. Chem. 2003, 47, 179–185. [Google Scholar] [CrossRef]
- Chirachanchai, S.; Rungsimanon, T.; Phongtamrag, S.; Miyata, M.; Laobuthee, A. Selective Macrocyclization: A Model Case Study from N,N-bis(2-hydroxy-3,5-dimethylbenzyl)alkylamine. Tetrahedron 2009, 65, 5855–5861. [Google Scholar] [CrossRef]
- Chirachanchai, S.; Laobuthee, A.; Phongtamrag, S. Self termination of ring opening reaction of p-substituted phenol-based benzoxazines: An obstructive effect via intramolecular hydrogen bond. J. Heterocycl. Chem. 2009, 46, 714–721. [Google Scholar] [CrossRef]
- Kaewvilai, A.; Rungsimanon, T.; Koonsaeng, N.; Chirachanchai, S.; Laobuthee, A. Branching structures of alkali metal ion responsive supramolecules based on symmetric structures of ,N-bis(5-alkyl-2-hydroxybenzyl)methylamine. Asian J. Chem. 2010, 22, 7628–7640. [Google Scholar]
- Holly, F.W.; Cope, A.C. Condensation Products of Aldehydes and Ketones with o-Aminobenzyl Alcohol and o-Hydroxybenzylamine. J. Am. Chem. Soc. 1944, 66, 1875. [Google Scholar] [CrossRef]
- Phongtamrug, S. Study on the benzoxazine monomers and their application for ion extraction material. Master Degree Thesis in Polymer Science. The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand, 1998. [Google Scholar]
- Pedersen, C.J. Cyclic Polyesters and Their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 1009–1020. [Google Scholar] [CrossRef]
- Pedersen, C.J.; Frensdorff, H.K. Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. Engl. 1972, 11, 16. [Google Scholar] [CrossRef]
- Rakthin, T.; Veranitisagul, C.; Koonsaeng, N.; Traversa, E.; Laobuthee, A. Preparation of Ceria Powder via Metal-Organic Complex Method. In Proceedings of Pure and Applied Chemistry International Conference: Chemistry for Sufficiency and Sustainability, Amsterdam, The Netherlands, 22-23 February 2008; Parasuk, W., Ed.; Chemical Society of Thailand: Bangkok, Thailand, 2008; pp. 312–316. [Google Scholar]
- Veranitisagul, C.; Kaewvilai, A.; Sangngern, S.; Wattanathana, W.; Suramitr, S.; Koonsaeng, N.; Laobuthee, A. Novel Recovery of Nano-Structured Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes via Thermal Decomposition. Int. J. Mol. Sci. 2011, 12, 4365–4377. [Google Scholar] [CrossRef]
- Wattanathana, W.; Lakkham, A.; Kaewvilai, A.; Koonsaeng, N.; Laobuthee, A.; Veranitisagul, C. Preliminary Study of Pd/CeO2 Derived from Cerium Complexes as Solid Support Catalysts for Hydrogenations Reaction in a Micro-reactor. Energy Procedia 2011, 9, 568–574. [Google Scholar] [CrossRef]
- Accelrys Inc Materials Studio 5.5, Accelrys: San Diego, CA, USA.
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, A Rule-Based Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024. [Google Scholar] [CrossRef]
- Rappé, A.K.; Colwell, K.S.; Casewit, C.J. Application of a Universal Force Field to Metallic Complexes. Inorg. Chem. 1993, 32, 3438. [Google Scholar] [CrossRef]
- Rappé, A.K.; Goddard, W.A. Charge equilibration for molecular dynamics simulations. J. Phys. Chem. 1991, 95, 3358–3363. [Google Scholar] [CrossRef]
- Casewit, C.J.; Colwell, K.S.; Rappé, A.K. Application of a Universal Force Field to Organic Molecules. J. Am. Chem. Soc. 1992, 114, 10035. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaewvilai, A.; Rujitanapanich, S.; Wattanathana, W.; Veranitisagul, C.; Suramitr, S.; Koonsaeng, N.; Laobuthee, A. The Effect of Alkali and Ce(III) Ions on the Response Properties of Benzoxazine Supramolecules Prepared via Molecular Assembly. Molecules 2012, 17, 511-526. https://doi.org/10.3390/molecules17010511
Kaewvilai A, Rujitanapanich S, Wattanathana W, Veranitisagul C, Suramitr S, Koonsaeng N, Laobuthee A. The Effect of Alkali and Ce(III) Ions on the Response Properties of Benzoxazine Supramolecules Prepared via Molecular Assembly. Molecules. 2012; 17(1):511-526. https://doi.org/10.3390/molecules17010511
Chicago/Turabian StyleKaewvilai, Attaphon, Sawittree Rujitanapanich, Worawat Wattanathana, Chatchai Veranitisagul, Songwut Suramitr, Nattamon Koonsaeng, and Apirat Laobuthee. 2012. "The Effect of Alkali and Ce(III) Ions on the Response Properties of Benzoxazine Supramolecules Prepared via Molecular Assembly" Molecules 17, no. 1: 511-526. https://doi.org/10.3390/molecules17010511