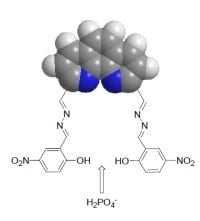
Synthesis and Binding Ability of Molecular Probes Based on a Phenanthroline Derivative: Theory and Experiment
Abstract
:1. Introduction

2. Results and Discussion
2.1. UV-Vis Titration


2.2. Fluorescence Response
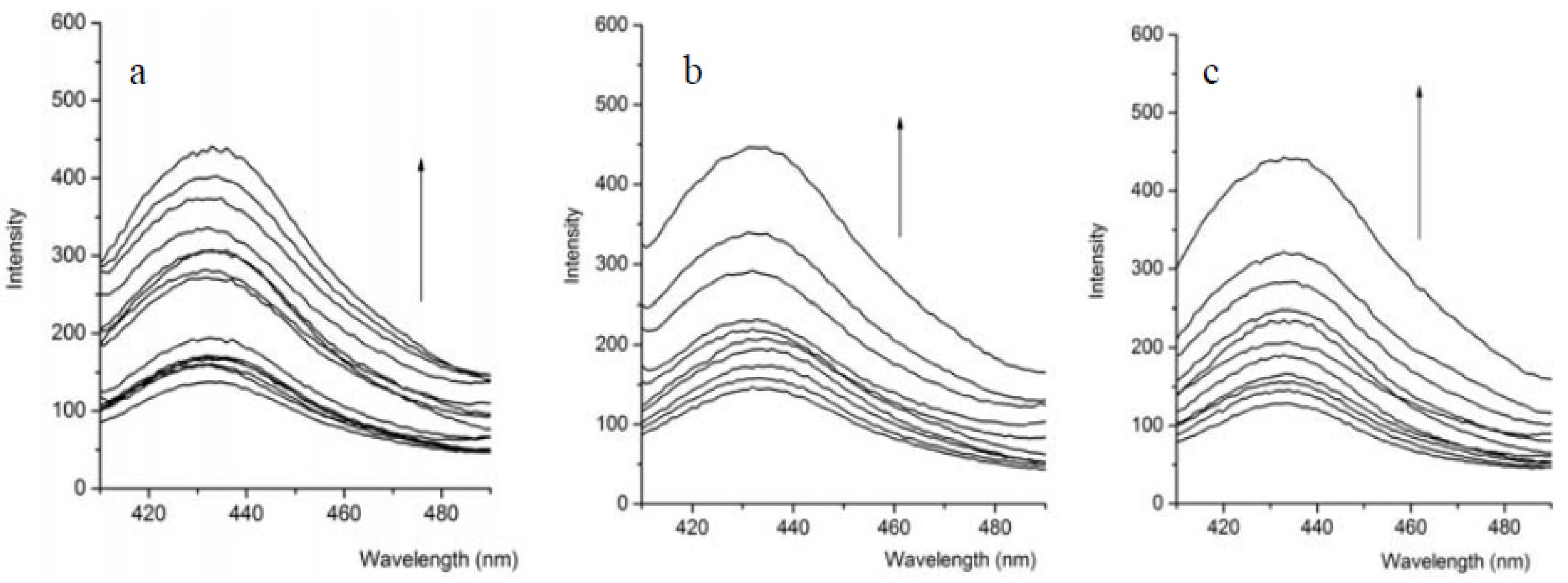
2.3. Binding Constant
| Anion a | Ks(1) | |
|---|---|---|
| H2PO4− | Absorption | (1.01 ± 0.08) × 105 |
| Emission | (1.67 ± 0.22) × 105 | |
| AcO− | Absorption | (3.43 ± 0.04) × 104 |
| Emission | (2.89 ± 0.16) × 104 | |
| F− | Absorption | (7.88 ± 0.11) × 104 |
| Emission | (6.57 ± 0.35) × 104 |
2.4. 1H-NMR Titration

2.5. Theoretical Investigation


3. Experimental
General
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wenzel, M.; Hiscock, J.R.; Gale, P.A. Anion receptor chemistry: Highlights from 2010. Chem. Soc. Rev. 2012, 41, 480–520. [Google Scholar]
- Kado, S.; Otani, H.; Nakahara, Y.; Kimura, K. Highly selective recognition of acetate and bicarbonate by thiourea-functionalised inverse opal hydrogel in aqueous solution. Chem. Commun. 2013, 49, 886–888. [Google Scholar] [CrossRef]
- Santos-Figueroa, L.E.; Moragues, M.E.; Climent, E.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar]
- Spence, G.T.; Chan, C.; Szemes, F.; Beer, P.D. Anion binding induced conformational changes exploited for recognition, Sensing and pseudorotaxane disassembly. Dalton Trans. 2012, 41, 13474–13485. [Google Scholar]
- Sharma, H.; Guadalupe, H.J.; Narayanan, J.; Hofeld, H.; Pandiyan, T.; Singh, N. Pyridyl- and benzimidazole-based ruthenium(III) complex for selective chloride recognition through fluorescence spectroscopy. Anal. Methods 2013, 5, 3880–3887. [Google Scholar] [CrossRef]
- Vinodh, M.; Alipour, F.H.; Mohamod, A.A.; Al-Azemi, T.F. Molecular assemblies of porphyrins and macrocyclic receptors: Recent developments in their synthesis and applications. Molecules 2012, 17, 11763–11799. [Google Scholar] [CrossRef]
- Li, Y.; Ashizawa, M.; Uchida, S.; Michinobu, T. Colorimetric sensing of cations and anions by clicked polystyrenes bearing side chain donor–acceptor chromophores. Polym. Chem. 2012, 3, 1996–2005. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, S.K.; Yoon, J. Revisit to imidazolium receptors for the recognition of anions: Highlighted research during 2006–2009. Chem. Soc. Rev. 2010, 39, 1457–1466. [Google Scholar] [CrossRef]
- Shang, X.F.; Li, X.J.; Xi, N.K.; Zhai, Y.T.; Zhang, J.L.; Xu, X.F. Theory and experiment: Recognition properties of chemosensor containing ruthenium(II) system in water solution. Sensor. Actuat. B Chem. 2011, 160, 1112–1119. [Google Scholar] [CrossRef]
- Ojida, A.; Mito-oka, Y.; Sada, K.; Hamachi, I. Molecular recognition and fluorescence sensing of monophosphorylated peptides in aqueous solution by bis(zinc(II)−dipicolylamine)-based artificial receptors. J. Am. Chem. Soc. 2004, 26, 2454–2463. [Google Scholar]
- Yang, Z.; Zhang, K.; Gong, F.; Li, S.; Chen, J.; Ma, J.S.; Sobenina, L.N.; Mikhaleva, A.I.; Trofimov, B.A.; Yang, G. A highly selective fluorescent sensor for fluoride anion based on pyrazole derivative: Naked eye “no–yes” detection. J. Photochem. Photobiol. A-Chem. 2011, 217, 29–34. [Google Scholar] [CrossRef]
- Jesús Seguí, M.; Lizondo-Sabater, J.; Benito, A. A new ion-selective electrode for anionic surfactants. Talanta 2007, 71, 333–338. [Google Scholar] [CrossRef]
- Tavallali, H.; Rad, G.D.; Parhami, A.; Abbasiyan, E. A new application of bromopyrogallol red as a selective and sensitive competition assay for recognition and determination of acetate anion in DMSO/water media. Dyes Pigm. 2012, 94, 541–547. [Google Scholar] [CrossRef]
- Huang, W.W.; Lin, H.; Lin, H.K. Fluorescent acetate-sensing in aqueous solution. Sensor. Actuat. B Chem. 2011, 153, 404–408. [Google Scholar] [CrossRef]
- Goswami, S.; Chakrabarty, R. Cu (II) complex of an abiotic receptor as highly selective fluorescent sensor for acetate. Tetrahedron Lett. 2009, 50, 5994–5997. [Google Scholar] [CrossRef]
- Misra, A.; Shahid, M.; Dwivedi, P. An efficient thiourea-based colorimetric chemosensor for naked-eye recognition of fluoride and acetate anions: UV–vis and 1H-NMR studies. Talanta 2009, 80, 532–538. [Google Scholar] [CrossRef]
- Huang, W.W.; Li, Y.; Lin, H.; Lin, H.K. A novel acetate selective chromogenic chemosensor based on phenanthroline. Spectrochim. Acta A-Mol. Biomol. Spectrosc. 2012, 86, 437–442. [Google Scholar] [CrossRef]
- Kondo, S.I.; Fukuda, A.; Yamamura, T.; Tanaka, R.; Unno, M. Anion recognition by a disiloxane-1, 3-diol in organic solvents. Tetrahedron Lett. 2007, 48, 7946–7949. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Q.L.; Jiang, Q.Q.; Jiang, Q.J.; Jiang, Y.B. Anion-triggered substituent-dependent conformational switching of salicylanilides. New hints for understanding the inhibitory mechanism of salicylanilides. J. Org. Chem. 2007, 72, 9947–9953. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, S.; Lee, K.H.; Yoon, J. A highly selective fluorescent chemosensor for dihydrogen phosphate via unique excimer formation and PET mechanism. Tetrahedron Lett. 2007, 48, 3797–3800. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Harada, J.; Fujiwara, T.; Ogawa, K. Crucial role of fluorescence in the solid-state thermochromism of salicylideneanilines. J. Am. Chem. Soc. 2007, 129, 16216–16221. [Google Scholar] [CrossRef]
- Lin, Z.H.; Zhao, Y.G.; Duan, C.Y.; Zhang, B.G.; Bai, Z.P. A highly selective chromo-and fluorogenic dual responding fluoride sensor: naked-eye detection of F− ion in natural water via a test paper. Dalton Trans. 2006, 30, 3678–3684. [Google Scholar]
- Liu, Y.; Han, B.H.; Zhang, H.Y. Spectroscopic studies on molecular recognition of modified cyclodextrins. Curr. Org. Chem. 2004, 8, 35–46. [Google Scholar] [CrossRef]
- Liu, Y.; You, C.C.; Zhang, H.Y. Supramolecular Chemistry; Nankai University Publication: Tianjin, China, 2001. [Google Scholar]
- Bourson, J.; Pouget, J.; Valeur, B. Ion-responsive fluorescent compounds. 4. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza-and diaza-crown ethers. J. Phys. Chem. 1993, 97, 4552–4557. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Revision A.1; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Sample Availability: Sample of compound 1 is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shang, X.; Wang, Y.; Wei, X.; Fu, Z.; Zhang, J.; Xu, X. Synthesis and Binding Ability of Molecular Probes Based on a Phenanthroline Derivative: Theory and Experiment. Molecules 2013, 18, 14840-14848. https://doi.org/10.3390/molecules181214840
Shang X, Wang Y, Wei X, Fu Z, Zhang J, Xu X. Synthesis and Binding Ability of Molecular Probes Based on a Phenanthroline Derivative: Theory and Experiment. Molecules. 2013; 18(12):14840-14848. https://doi.org/10.3390/molecules181214840
Chicago/Turabian StyleShang, Xuefang, Yingling Wang, Xiaofang Wei, Zhiyuan Fu, Jinlian Zhang, and Xiufang Xu. 2013. "Synthesis and Binding Ability of Molecular Probes Based on a Phenanthroline Derivative: Theory and Experiment" Molecules 18, no. 12: 14840-14848. https://doi.org/10.3390/molecules181214840