Multicomponent Click Synthesis of New 1,2,3-Triazole Derivatives of Pyrimidine Nucleobases: Promising Acidic Corrosion Inhibitors for Steel
Abstract
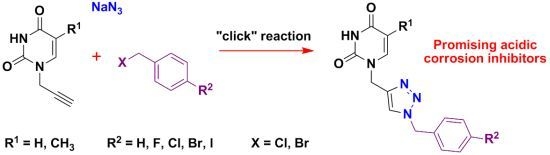
:1. Introduction
2. Results and Discussion
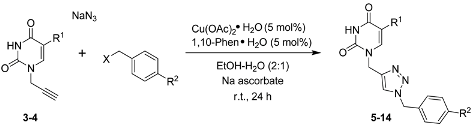
2.1. Synthesis

| Entry | Compound | R1 | R2 | X | Yield a (%) |
|---|---|---|---|---|---|
| 1 | 5 | H | H | Cl | 84 |
| 2 | 6 | H | F | Cl | 90 |
| 3 | 7 | H | Cl | Cl | 80 |
| 4 | 8 | H | Br | Br | 83 |
| 5 | 9 | H | I | Br | 81 |
| 6 | 10 | CH3 | H | Cl | 81 |
| 7 | 11 | CH3 | F | Cl | 90 |
| 8 | 12 | CH3 | Cl | Cl | 87 |
| 9 | 13 | CH3 | Br | Br | 83 |
| 10 | 14 | CH3 | I | Br | 85 |
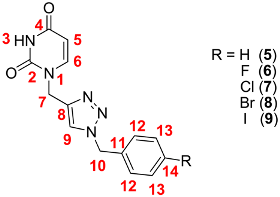
| 3-H | 5-H | 6-H | 7-H | 9-H | 10-H | 12-H | 13-H | 14-H | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-C | 4-C | 5-C | 6-C | 7-C | 8-C | 9-C | 10-C | 11-C | 12C | 13-C | 14-C | ||
| 5 | 11.29 | [a] | 7.71 | 4.89 | 8.10 | [a] | [a] | [a] | [a] | ||||
| 151.3 | 164.3 | 101.8 | 146.1 | 43.0 | 143.2 | 124.2 | 53.4 | 136.4 | 128.5 | 129.3 | 128.7 | ||
| 6 | 11.28 | 5.55 | 7.71 | 4.89 | 8.10 | 5.53 | 7.36 | 7.17 | --- | ||||
| 151.3 | 164.2 | 101.8 | 146.1 | 43.0 | 143.3 | 124.1 | 52.5 | 132.7 | 130.9 | 116.1 | 162.4 | ||
| 7 | 11.28 | 5.55 | 7.71 | 4.89 | 8.11 | 5.54 | 7.30 | 7.40 | --- | ||||
| 151.3 | 164.2 | 101.8 | 146.1 | 43.0 | 143.3 | 124.3 | 52.5 | 135.4 | 130.5 | 129.3 | 133.4 | ||
| 8 | 11.29 | 5.55 | 7.70 | 4.88 | 8.11 | 5.52 | 7.23 | 7.53 | --- | ||||
| 151.3 | 164.3 | 101.8 | 146.1 | 43.0 | 143.3 | 124.3 | 52.6 | 135.8 | 130.8 | 132.2 | 122.0 | ||
| 9 | 11.28 | 5.50 | 7.70 | 4.88 | 8.10 | 5.50 | 7.08 | 7.70 | --- | ||||
| 151.3 | 164.3 | 101.8 | 146.1 | 43.0 | 143.2 | 124.3 | 52.7 | 136.2 | 130.8 | 138.1 | 95.1 |
2.2. Corrosion Inhibition Efficiencies
| 3-H | 6-H | 7-H | 8-H | 10-H | 11-H | 13-H | 14-H | 15-H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-C | 4-C | 5-C | 6-C | 7-C | 8-C | 9-C | 10-C | 11-C | 12-C | 13-C | 14-C | 15-C | ||
| 10 | 11.28 | 7.59 | 4.85 | 1.71 | 8.10 | 5.54 | [a] | [a] | [a] | |||||
| 151.2 | 164.8 | 109.4 | 141.8 | 42.8 | 12.5 | 143.4 | 124.2 | 53.3 | 136.5 | 128.5 | 129.3 | 128.7 | ||
| 11 | 11.27 | 7.58 | 4.85 | 1.71 | 8.10 | 5.53 | 7.36 | 7.17 | --- | |||||
| 151.2 | 164.8 | 109.4 | 141.7 | 42.8 | 12.5 | 143.4 | 124.1 | 52.5 | 132.7 | 130.9 | 116.1 | 162.4 | ||
| 12 | 11.27 | 7.59 | 4.85 | 1.71 | 8.10 | 5.54 | 7.30 | 7.40 | --- | |||||
| 151.2 | 164.8 | 109.4 | 141.7 | 42.8 | 12.5 | 143.4 | 124.2 | 52.5 | 135.5 | 130.5 | 129.3 | 133.4 | ||
| 13 | 11.30 | 7.61 | 4.87 | 1.73 | 8.13 | 5.55 | 7.26 | 7.561 | --- | |||||
| 151.3 | 164.8 | 109.4 | 141.8 | 42.8 | 12.5 | 143.5 | 124.3 | 52.6 | 135.9 | 130.8 | 32.2 | 122.0 | ||
| 14 | 11.29 | 7.60 | 4.87 | 1.74 | 8.11 | 5.53 | 7.10 | 7.72 | --- | |||||
| 151.3 | 164.8 | 109.4 | 141.7 | 42.8 | 12.5 | 143.4 | 124.3 | 52.8 | 136.2 | 130.9 | 138.1 | 95.1 |

| Compound | Rs/Ω cm2 | Rct/Ω cm2 | Cdl/μF cm2 | IE/% |
|---|---|---|---|---|
| Blank | 0.8 | 30 | 310 | --- |
| 5 | 2.5 | 435 | 39 | 93.1 |
| 6 | 1.3 | 681 | 43 | 95.6 |
| 7 | 1.1 | 725 | 50 | 95.9 |
| 8 | 1.0 | 770 | 18 | 96.1 |
| 9 | 1.7 | 425 | 70 | 92.9 |
| 10 | 1.5 | 306 | 19 | 90.2 |
| 11 | 1.4 | 600 | 29 | 95.0 |
| 12 | 1.4 | 599 | 56 | 95.0 |
| 13 | 1.5 | 600 | 54 | 95.0 |
| 14 | 1.3 | 588 | 49 | 94.9 |
3. Experimental
3.1. General
3.2. Product Synthesis and Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Ganesh, A. Potential biological activity of 1,4-sustituted-1H-[1,2,3]triazoles. Int. J. Chem. Sci. 2013, 11, 573–578. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Hiusgen cycloaddition process: Copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 1, 51–68. [Google Scholar]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (cuaac) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef]
- Lallana, E.; Riguera, R.; Fernandez-Mejia, E. Reliable and efficient procedures for the conjugation of biomolecules through Huisgen azide-alkyne cycloadditions. Angew. Chem. Int. Ed. 2011, 50, 8794–8804. [Google Scholar] [CrossRef]
- Pedersen, D.S.; Abell, A. 1,2,3-Triazoles in peptidomimetic chemistry. Eur. J. Org. Chem. 2011, 2399–2411. [Google Scholar] [CrossRef]
- Efthymiou, T.; Gong, W.; Desaulniers, J.-P. Chemical architecture and applications of nucleic acid derivatives containing 1,2,3-triazole funtionalities synthesized via click chemistry. Molecules 2012, 17, 12665–12703. [Google Scholar] [CrossRef]
- Chittepu, P.; Sirivolu, V.R.; Seela, F. Nucleosides and oligonucleotides containing 1,2,3-triazole residues with nucleobase tethers: Synthesis via azide-alkyne ‘click’ reaction. Bioorg. Med. Chem. 2008, 16, 8427–8439. [Google Scholar] [CrossRef]
- Lolk, L.; Pøhlsgaard, J.; Jepsen, A.S.; Hansen, L.H.; Nielsen, H.; Steffansen, S.I.; Sparving, L.; Nielsen, A.B.; Vester, B.; Nielsen, P. A click chemistry approach to pleuromutilin conjugates with nucleosides or acyclic nucleoside derivatives and their binding to the bacterial ribosome. J. Med. Chem. 2008, 51, 4957–4967. [Google Scholar] [CrossRef]
- Ganesen, M.; Muraleedharan, K.M. Synthesis of β-Hydroxyphosphonate and 1,2-Dihydroxy Acyclic Nucleoside Analogs via 1,3-Dipolar Cycloaddition Strategy. Nucleos. Nucleot. Nucleic Acids 2010, 29, 91–96. [Google Scholar] [CrossRef]
- Trakossas, S.; Coutouli-Argyropoulou, E.; Hadjipavlou-Litina, D.J. Synthesis of modified triazole nucleosides possessing one or two base moieties via a click chemistry approach. Tetrahedron Lett. 2011, 52, 1673–1676. [Google Scholar] [CrossRef]
- Jørgensen, A.S.; Shaikh, K.I.; Enderlin, G.; Ivarsen, E.; Kumar, S.; Nielsen, P. The synthesis of double-headed nucleosides by the cuaac reaction and their effect in secondary nucleic acid structures. Org. Biomol. Chem. 2011, 9, 1381–1388. [Google Scholar] [CrossRef]
- Parmenopoulou, V.; Chatzileontiadou, D.S.M.; Manta, S.; Bougiatioti, S.; Maragozidis, P.; Gkaragkouni, D.-N.; Kaffesaki, E.; Kantsadi, A.L.; Skamnaki, V.T.; Zographos, S.E.; et al. Triazole pyrimidine nucleosides as inhibitors of ribonuclease A. Synthesis, Biochemical, and structural evaluation. Bioorg. Med. Chem. 2012, 20, 7184–7193. [Google Scholar] [CrossRef]
- Krim, J.; Taourirte, M.; Grünewald, C.; Krstic, I.; Engels, J.W. Microwave-assisted click chemistry for nucleoside functionalization: useful derivatives for analytical and biological applications. Synthesis 2013, 45, 396–405. [Google Scholar] [CrossRef]
- Elayadi, H.; Smietana, M.; Pannecouque, C.; Leyssen, P.; Neyts, J.; Vasseur, J.-J.; Lazrek, H.B. Straightforward synthesis of triazoloacyclonucleotide phosphonates as potential hcv inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7365–7368. [Google Scholar]
- Głowacka, I.E.; Balzarini, J.; Wróblewski, A.E. Design, Synthesis, Antiviral, and cytotoxic evaluation of novel phosphonylated 1,2,3-triazoles as acyclic nucleotide analogues. Nucleos. Nucleot. Nucleic Acids 2012, 31, 293–318. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Balzarini, J.; Głowacka, I.E. Design, Synthesis, Antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur. J. Med. Chem. 2012, 47, 501–509. [Google Scholar] [CrossRef]
- Diab, S.A.; De Schutter, C.; Muzard, M.; Plantier-Royon, R.; Pfund, E.; Lequeux, T. Fluorophosphonylated nucleoside derivatives as new series of thymidine phosphorylase multisubstrate inhibitors. J. Med. Chem. 2012, 55, 2758–2768. [Google Scholar] [CrossRef]
- Głowacka, I.E.; Balzarini, J.; Wróblewski, A.E. Synthesis and biological evaluation of novel 1,2,3-triazolonucleotides. Arch. Pharm. Chem. 2013, 346, 278–291. [Google Scholar] [CrossRef]
- Soltani Rad, M.N.; Asrari, Z.; Behrouz, S.; Hakimelahi, G.H.; Khalafi-Nezhad, A. ‘Click Synthesis’ of 1H-1,2,3-Triazolyl-Based Oxiconazole (=(1Z)-1-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-yl)ethanone O-[(2,4-Dichlorophenyl)methyl] oxime) Analogs. Helv. Chim. Acta 2011, 94, 2194–2206. [Google Scholar] [CrossRef]
- Kramer, R.A.; Bleicher, K.H.; Wennemers, H. Design and synthesis of nucleoproline amino acids for the straightforward preparation of chiral and conformationally constrained nucleopeptides. Helv. Chim. Acta 2012, 95, 2621–2634. [Google Scholar] [CrossRef]
- Wang, P.; Leung, C.-H.; Ma, D.-L.; Lu, W.; Che, C.-M. Organoplatinum(II) complexes with nucleobases motifs as inhibitors of human topoisomerase II catalytic activity. Chem. Asian J. 2010, 5, 2271–2280. [Google Scholar] [CrossRef]
- Noel, O.; Xie, J. Synthesis of nucleo aminooxy acid derivatives. Synthesis 2013, 45, 134–140. [Google Scholar]
- Lu, J.; Hu, J.; Song, Y.; Ju, Y. A new dual-responsive organogel based on uracil-appended glycyrrhetinic acid. Org. Lett. 2011, 13, 3372–3375. [Google Scholar] [CrossRef]
- Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Maldonado-Carmona, N.; Espinoza-Vázquez, A.; Plomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Synthesis of New 1,2,3-Triazole Derivatives of Uracil and Thymine with Potential Inhibitory Activity against Acidic Corrosion of Steels. Molecules 2013, 18, 4613–4627. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thiehylmethyl)-4-amino-1,2,4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Ouici, H.B.; Benali, O.; Harek, Y.; Larabi, L.; Hammouti, B.; Guendouzi, A. Inhibition of mild steel corrosion in 5% HCl solution by 5-(2-hydroxyphenyl)-1,2,4-triazole-3-thione. Res. Chem. Intermed. 2013, 39, 2777–2793. [Google Scholar]
- John, S.; Joseph, A. Electro analytical, surface morphological and theoretical studies on the corrosion inhibition behavior of different 1,2,4-triazole precursors on mild steel in 1M hydrochloric acid. Mater. Chem. Phys. 2012, 133, 1083–1091. [Google Scholar] [CrossRef]
- Ansari, K.R.; Yadav, D.K.; Ebenso, E.E.; Quraishi, M.A. Novel and effective pyridyl substituted 1,2,4-triazole as corrosion inhibitor for mild steel in acid solution. Int. J. Electrochem. Sci. 2012, 7, 4780–4799. [Google Scholar]
- Mert, B.D.; Mert, M.E.; Kardaş, G.; Yazıcı, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, S.; Quan, H.; Huang, Z.; Xu, S. Synthesis and corrosion inhibition performance of alkyl triazole derivatives. Res. Chem. Intermed. 2013. [Google Scholar] [CrossRef]
- Deng, Q.; Ding, N.-N.; Wei, X.-L.; Cai, L.; He, X.-P.; Long, Y.-T.; Chen, G.-R.; Chen, K. Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.-W.; Ding, N.-N.; Chen, B.-Q.; He, X.-P.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R. Novel triazolyl bis-amino acid derivatives readily synthesized via click chemistry as potential corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 57, 220–227. [Google Scholar]
- Zhang, H.-L.; He, X.-P.; Deng, Q.; Long, Y.-T.; Chen, G.-R.; Chen, K. Research on the structure-surface adsorptive activity relationships of triazolyl glycolipid derivatives for mild steel in HCl. Carbohydr. Res. 2012, 354, 32–39. [Google Scholar] [CrossRef]
- Malki Alaoui, L.; Hammouti, B.; Bellaouchou, A.; Benbachir, A.; Guenbour, A.; Kertit, S. Corrosion inhibition and adsorption properties of 3-amino-1,2,3-triazole on mild steel in H3PO4. Pharm. Chem. 2011, 3, 353–360. [Google Scholar]
- Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Angeles-Beltrán, D.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Herrera-Hernández, H. Electrochemical impedance evaluation of uracil and thymine pyrimidine derivatives and its nucleosides compounds as a non-toxic corrosion inhibitors of steels in 1M HCl. ECS Trans. 2011, 36, 217–228. [Google Scholar]
- Lazrek, H.B.; Taourirte, M.; Oulih, T.; Kabbaj, Y.; Barascut, J.L.; Imbach, J.L.; Almasoudi, N.A.; Pfleiderer, W. Synthesis of 3'-deoxy-3' and 5'-deoxy-5'-[4-(purin-9-yl/pyrimidin-1-yl)methyl-1,2,3-triazol-1-yl]thymidine via 1,3-dipolar cycloaddition. Nucleo. Nucleot. 1997, 16, 1073–1077. [Google Scholar] [CrossRef]
- Lazrek, H.B.; Taourirte, M.; Oulih, T.; Barascut, J.L.; Imbach, J.L.; Pannecouque, C.; Witrouw, M.; de Clercq, E. Synthesis and anti-hiv activity of new modified 1,2,3-triazole acyclonucleosides. Nucleo. Nucleot. Nucleic Acids 2001, 20, 1949–1960. [Google Scholar] [CrossRef]
- Hakimelahi, G.H.; Gassanov, G.S.; Hsu, M.-H.; Hwu, J.R.; Hakimelahi, S. A novel approach towards studying non-genotoxic enediynes as potential anticancer therapeutics. Bioorg. Med. Chem. 2002, 10, 1321–1328. [Google Scholar] [CrossRef]
- Ubasawa, M.; Takashima, H.; Sekiya, K. A convenient one-pot synthesis of acyclonucleosides. Chem. Pharm. Bull. 1995, 43, 142–143. [Google Scholar] [CrossRef]
- Lindsell, W.E.; Murray, C.; Preston, P.N.; Woodman, T.A.J. Synthesis of 1,3-diynes in the purine, pyrimidine, 1,3,5-triazine and acridine series. Tetrahedron 2000, 56, 1233–1245. [Google Scholar] [CrossRef]
- Hamon, F.; Violeau, B.; Turpin, F.; Bellot, M.; Bouteiller, L.; Djedaini-Pilard, F.; Len, C. Potential supramolecular cyclodextrin dimers using nucleobase pairs. Synlett 2009, 2875–2879. [Google Scholar]
- Appukkuttan, P.; Dehaen, W.; Fokin, V.V.; van der Eycken, E. A microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper(i)-catalyzed three-component reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Basu, D.; Rambabu, Ch. Three-component coupling of alkynes, Baylis-Hillman adducts and sodium azide: A new synthesis of substituted triazoles. Tetrahedron Lett. 2006, 47, 3059–3063. [Google Scholar] [CrossRef]
- Malnuit, V.; Duca, M.; Manout, A.; Bougrin, K.; Benhida, R. Tandem azide-alkyne 1,3-dipolar cycloaddition/electrophilic addition: A concise three-component route to 4,5-disubstituted triazolyl-nucleosides. Synlett 2009, 2123–2128. [Google Scholar]
- Zhang, J.; Wu, J.; Shen, L.; Jin, G.; Cao, S. Novel synthesis of difluoromethyl-containing 1,4-disubstituted 1,2,3-triazoles via a click-multicomponent reaction and desulfanylation strategy. Adv. Synth. Catal. 2011, 353, 580–584. [Google Scholar] [CrossRef]
- Mendoza-Espinosa, D.; Negrón-Silva, G.E.; Lomas-Romero, L.; Gutiérrez-Carrillo, A.; Soto-Castro, D. Efficient multicomponent synthesis of mono-, bis-, and tris-1,2,3-triazoles supported by hydroxybenzene scaffolds. Synthesis 2013, 45, 2431–2437. [Google Scholar] [CrossRef]
- Creary, X.; Anderson, A.; Brophy, C.; Crowell, F.; Funk, Z. Method for assigning structure of 1,2,3-triazoles. J. Org. Chem. 2012, 77, 8756–8761. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Zhang, Y.; Wang, P.; Zhang, J. Theoretical evaluation of inhibition performance of purine corrosion inhibitors. Mol. Simulat. 2013, 39, 1034–1041. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, D.Y.; Negrón-Silva, G.; Angeles-Beltrán, D.; Palomar-Pardavé, M.; Romero-Romo, M.; Herrera-Hernández, H. Adenine and guanine derivative bases of purines and their corresponding nucleosides as corrosion inhibitors in 1M hydrochloric acid. ECS Trans. 2011, 36, 179–185. [Google Scholar]
- Yan, Y.; Li, W.; Cai, L.; Hou, B. Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1M HCl solution. Electrochim. Acta 2008, 53, 5953–5960. [Google Scholar] [CrossRef]
- Elayadi, H.; Ali, M.A.; Mehdi, A.; Lazrek, H.B. Nanoscrystalline CuO: Synthesis and application as an efficient catalyst for the preparation of 1,2,3-triazole acyclic nucleosides via 1,3-dipolar cycloaddition. Catal. Commun. 2012, 26, 155–158. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3–14 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
González-Olvera, R.; Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Multicomponent Click Synthesis of New 1,2,3-Triazole Derivatives of Pyrimidine Nucleobases: Promising Acidic Corrosion Inhibitors for Steel. Molecules 2013, 18, 15064-15079. https://doi.org/10.3390/molecules181215064
González-Olvera R, Espinoza-Vázquez A, Negrón-Silva GE, Palomar-Pardavé ME, Romero-Romo MA, Santillan R. Multicomponent Click Synthesis of New 1,2,3-Triazole Derivatives of Pyrimidine Nucleobases: Promising Acidic Corrosion Inhibitors for Steel. Molecules. 2013; 18(12):15064-15079. https://doi.org/10.3390/molecules181215064
Chicago/Turabian StyleGonzález-Olvera, Rodrigo, Araceli Espinoza-Vázquez, Guillermo E. Negrón-Silva, Manuel E. Palomar-Pardavé, Mario A. Romero-Romo, and Rosa Santillan. 2013. "Multicomponent Click Synthesis of New 1,2,3-Triazole Derivatives of Pyrimidine Nucleobases: Promising Acidic Corrosion Inhibitors for Steel" Molecules 18, no. 12: 15064-15079. https://doi.org/10.3390/molecules181215064