Vasodilator Activity of the Essential Oil from Aerial Parts of Pectis brevipedunculata and Its Main Constituent Citral in Rat Aorta
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
| Compounds | a RI Lit | b RI | FRESH HD % | Identification |
|---|---|---|---|---|
| α-Pinene | 939 | 938 | 3.3 | RI, GCMS |
| Limonene | 1029 | 1032 | 4.5 | RI, GCMS |
| Longipenene epoxide | 1089 | 1186 | 1.4 | RI, GCMS |
| Nerol | 1233 | 1233 | 1.5 | RI, GCMS |
| Neral | 1247 | 1248 | 32.5 | RI, GCMS |
| Geraniol | 1276 | 1260 | 5.1 | RI, GCMS |
| Geranial | 1277 | 1278 | 49.2 | RI, GCMS |
| 1-Tridecene | 1293 | 1292 | 0.8 | RI, GCMS |
| β-Elemene | 1393 | 1389 | 0.7 | RI, GCMS |
| β-Farnesene | 1445 | 1418 | 0.2 | RI, GCMS |
| α-Cariophylene | 1457 | 1455 | 0.3 | RI, GCMS |
| Sum of identified peaks | 99.5% | |||
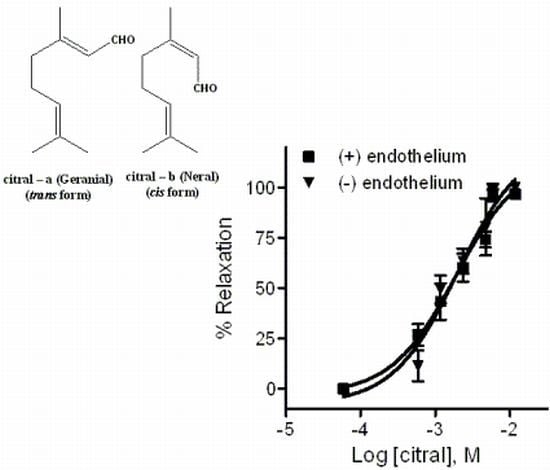
2.2. Pharmacology
2.2.1. Effects of EOPB and Citral on Vascular Smooth Muscle
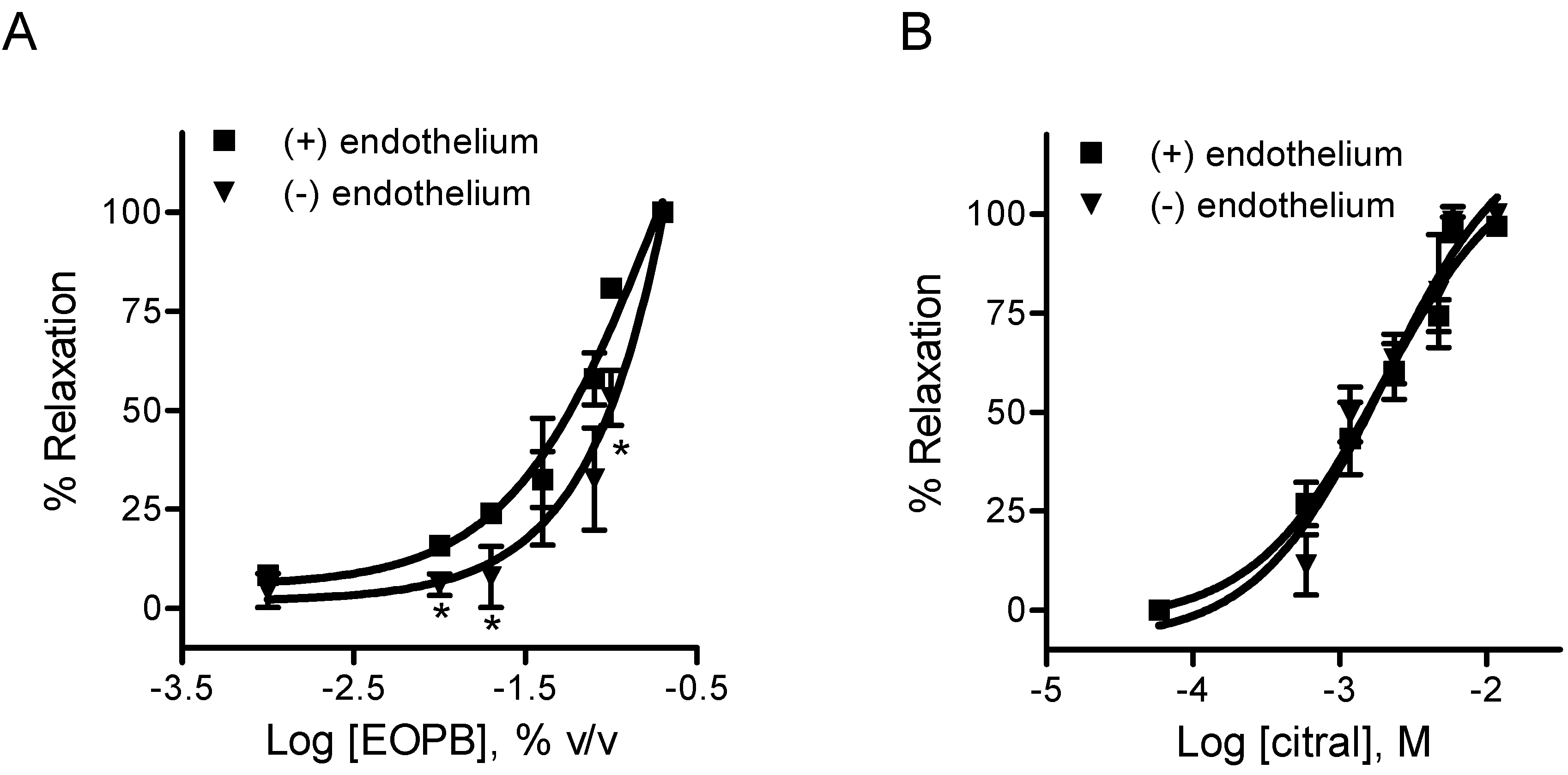
| IC50 | ||
|---|---|---|
| EOPB (%) | Citral (mM) | |
| With endothelium | 0.044 ± 0.006 | 1.42 ± 0.26 |
| Without endothelium | 0.093 ± 0.015 a | 1.33 ± 0.18 |
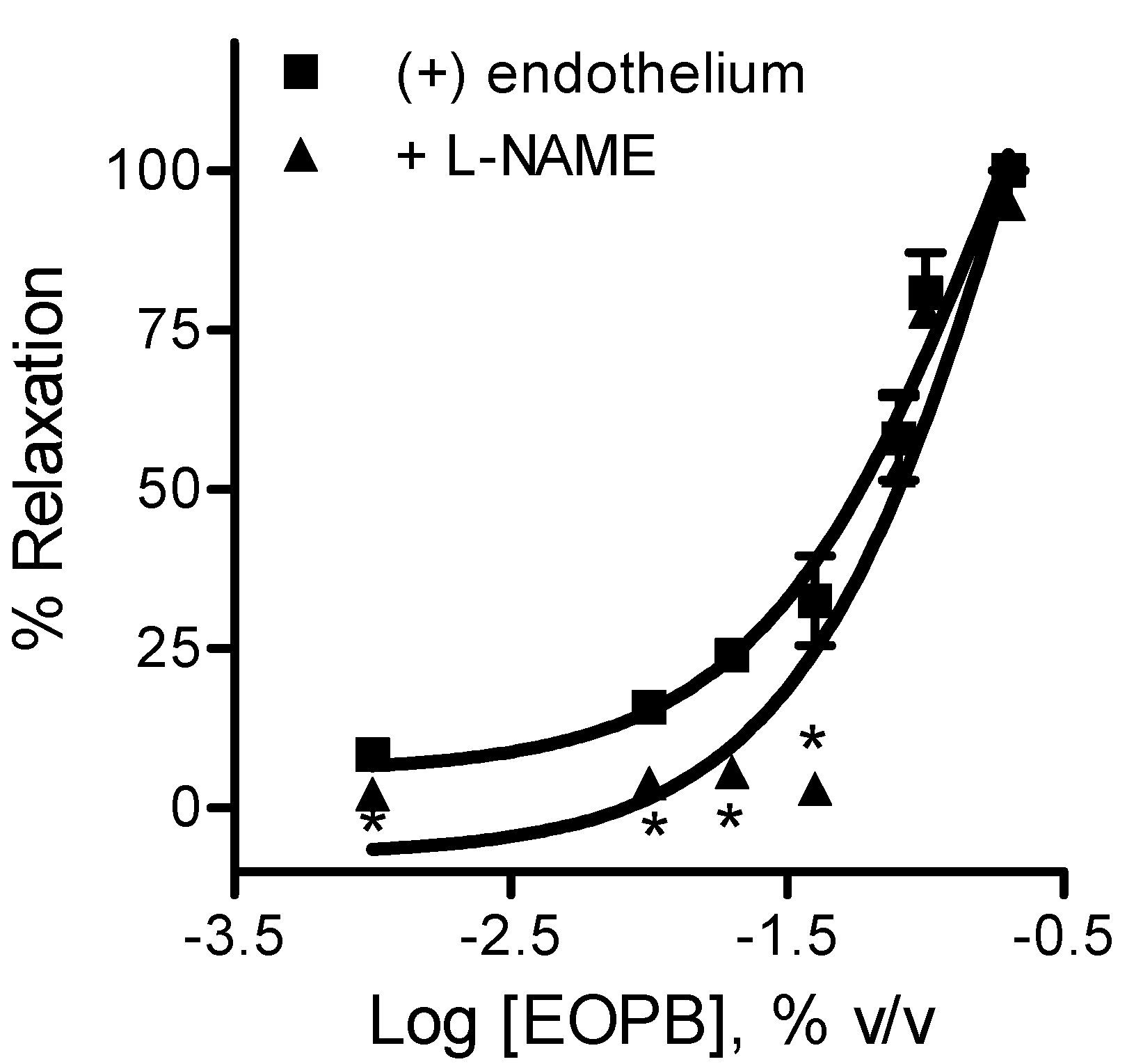
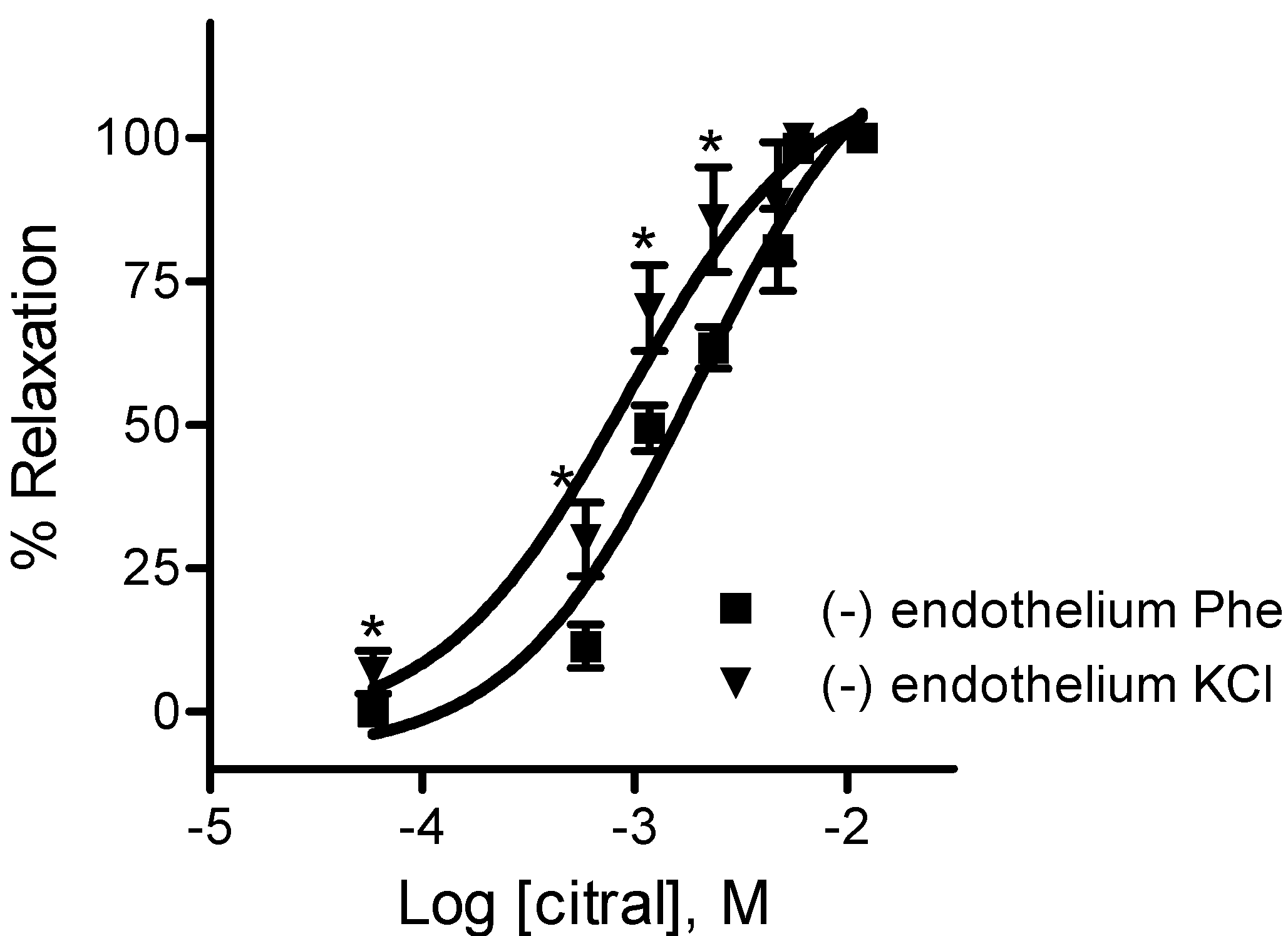
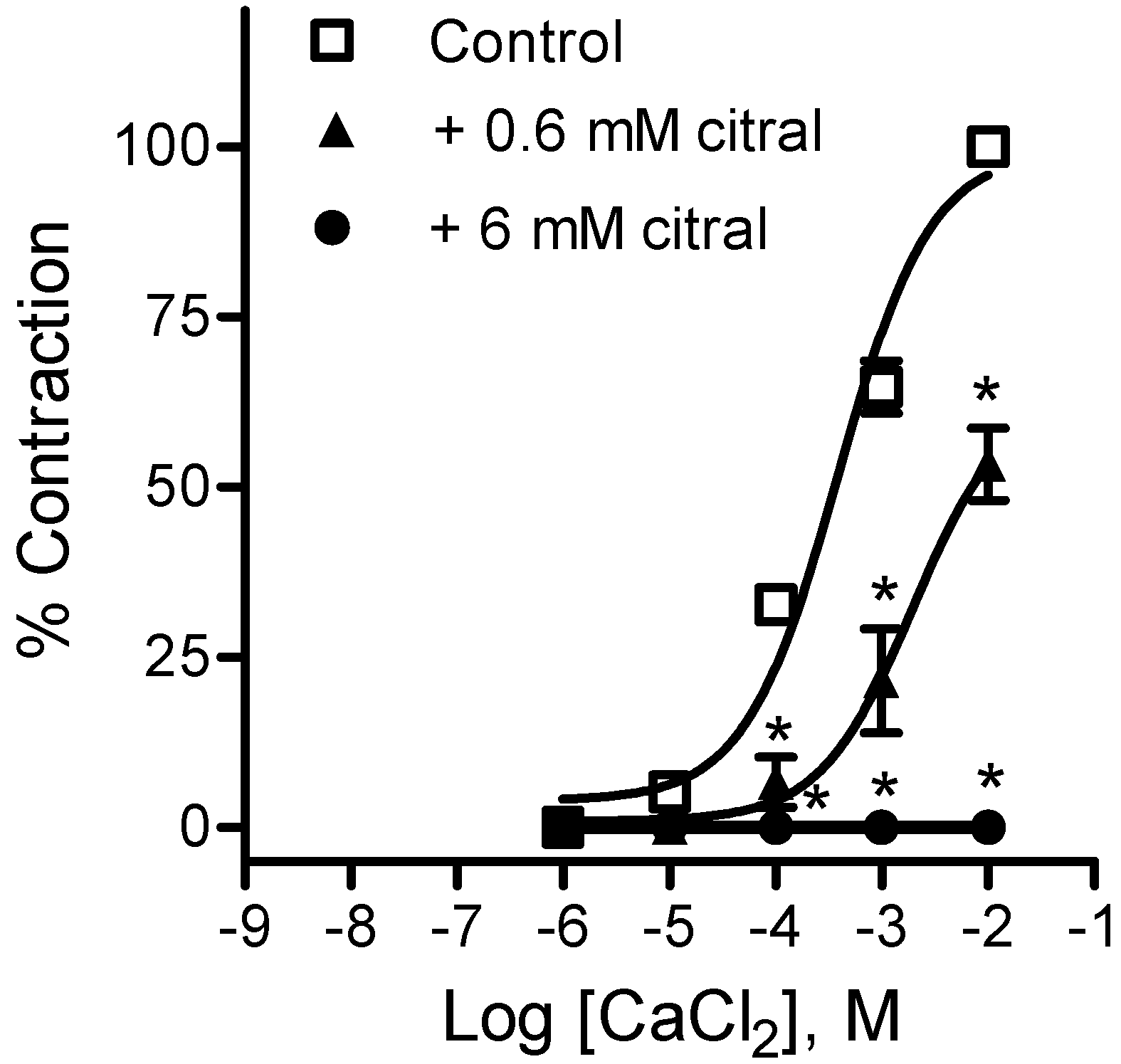
3. Experimental
3.1. Chemistry
3.1.1. Plant Material and Essential Oil Extraction
3.1.2. GC-FID Analysis
3.1.3. GC-MS Analysis
3.1.4. Countercurrent Chromatography Separation Procedure
3.1.4.1. Preparation of Two-Phase Solvent System and Sample Solutions
3.1.4.2. Apparatus and Citral Separation Procedure
3.2. Pharmacology
3.2.1. Preparation of Aortic Rings and Protocols
3.2.2. Compounds
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Keil, D.J. Two new species of Pectis (Asteraceae) from South America. Novon 2002, 12, 471–473. [Google Scholar] [CrossRef]
- Keil, D.J. New species of Pectis (Asteraceae) from the West Indies, Mexico and South America. Brittonia 1984, 36, 74–80. [Google Scholar] [CrossRef]
- Laferriere, J.E.; Keil, D.J. Pectis pimana (Asteraceae): A new species from Northwestern Mexico. Madrono 1991, 38, 195–197. [Google Scholar]
- Maia, J.G.S.; Silva, M.H.L.; Andrade, E.H.A. The essential oil of Pectis elongata Kunth occurring in north Brazil. Flavour. Frag. J. 2005, 20, 462–464. [Google Scholar] [CrossRef]
- Agra, M.F.; Freitas, P.F.; Barbosa-Filho, J.M. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Braz. J. Pharmacog. 2007, 17, 114–140. [Google Scholar]
- Soares, C.C.; Marques, T.M.; Rigolin, G.G.; Neis, E.; Friaça, A.M.V.; Silva, A.S. Atividade analgésica do extrato da Pectis jangadensis (S. Moore). Braz. J. Pharmacog. 2009, 19, 77–81. [Google Scholar]
- Oliveira, M.T.R.; Berbert, P.A. Efeito da temperatura do ar de secagem sobre o teor e a composição química do oleo essencial de Pectis brevipedunculata. Quim. Nova 2011, 34, 1200–1204. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, A.; Singh, P.; Dubey, N.K. Efficacy of Lippia alba (Mill.) N.E. Brown essential oil and its monoterpene aldehyde constituents against fungi isolated from some edible legume seeds and aflatoxin B1 production. Int. J. Food Microbiol. 2009, 135, 165–170. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement. Altern. Med. 2010, 10. [Google Scholar] [CrossRef]
- Demuner, A.J.; Barbosa, L.C.A.; Magalhães, C.G.; Silva, C.J.; Maltha, C.R.A.; Pinheiro, A.L. Seasonal variation in the chemical composition and antimicrobial activity of volatile oils of three species of Leptospermum (Myrtaceae) grown in brazil. Molecules 2011, 16, 1181–1191. [Google Scholar] [CrossRef]
- Kim, E.; Park, K. Fumigant antifungal activity of Myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Molecules 2012, 17, 10459–10469. [Google Scholar] [CrossRef]
- Moreira, F.V.; Bastos, J.F.A.; Blank, A.F.; Alves, P.B.; Santos, M.R.V. Chemical composition and cardiovascular effects induced by the essential oil of Cymbopogon citratus DC. Stapf, Poaceae, in rats. Braz. J. Pharmacog. 2010, 20, 904–909. [Google Scholar]
- Cha, J.H.; Lee, S.H.; Yoo, Y.S. Effects of aromatherapy on changes in the autonomic nervous system, aortic pulse wave velocity and aortic augmentation index in patientswith essential hypertension. J. Korean Acad. Nurs. 2010, 40, 705–713. [Google Scholar] [CrossRef]
- Devi, R.C.; Sim, S.M.; Ismail, R. Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol, IL, USA, 2007. [Google Scholar]
- Caluscusin, I.R.C. The effect of twice-a-day intake of lemon grass decoction among hypertensive individuals of barangay situbo, tampilisan, zamboanga del norte. Ph.D. Thesis, School of Medicine, Ateneo de Zamboanga University, Zamboanga City, Filipinas, April 2010. [Google Scholar]
- Carlini, E.A.; Contar, J.D.P.; Silva-Filho, A.R.; Silveira-Filho, N.G.; Frochtengarten, M.L.; Bueno, O.F. Pharmacology of lemongrass (Cymbopogon citratus Stapf). I. Effects of teas prepared from the leaves on laboratory animals. J. Ethnopharmacol. 1986, 17, 37–64. [Google Scholar] [CrossRef]
- Souza-Formigoni, M.L.; Lodder, H.M.; Gianotti-Filho, O.; Ferreira, T.M.; Carlini, E.A. Pharmacology of lemongrass (Cymbopogon citratus Stapf) II. Effects of daily two months administration in male and female rats and in offspring exposed “in utero”. J. Ethnopharmacol. 1986, 17, 65–74. [Google Scholar] [CrossRef]
- Viana, G.S.B.; Vale, T.G.; Pinho, R.S.N.; Matos, F.J.A. Anti-nociceptive effect of the essential oil from Cymbopogon citratus in mice. J. Ethnopharmacol. 2000, 70, 323–327. [Google Scholar] [CrossRef]
- Leite, J.R.; de Lourdes, V.; Seabra, M.; Maluf, E.; Assolant, K.; Suchecki, D.; Tufik, S.; Klepacz, S.; Calil, H.M.; Carlini, E.A. Pharmacology of lemongrass (Cymbopogon citratus Stapf). III. Assessment of eventual toxic, hypnotic and anxiolytic effects on humans. J. Ethnopharmacol. 1986, 17, 75–83. [Google Scholar] [CrossRef]
- Blanco, M.M.; Costa, C.A.; Freire, A.O.; Santos, J.G.; Costa, M. Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine 2009, 16, 265–270. [Google Scholar] [CrossRef]
- Vendruscolo, G.S.; Maris, S.; Rates, K.; Mentz, L.A. Chemical and pharmacologic data on medicinal plants used by the community of the Ponta Grossa neighborhood, Porto Alegre, Rio Grande do Sul, Brazil. Rev. Bras. Farmacogn. 2005, 15, 361–372. [Google Scholar]
- Omotade, I.O. Chemical profile and antimicrobial activity of Cymbopogon citratus leaves. J. Nat. Prod. 2009, 2, 98–103. [Google Scholar]
- Shimono, K.; Hiroaki, O.; Masato, S.; Kanae, S.; Shoji, K. Aromatic Antihypertensive Agent, and Method for Lowering Blood Pressure in Mammals. U.S. Patent 20100216891 A1, 26 August 2010. [Google Scholar]
- Karaki, H.; Ozaki, H.; Hori, M.; Mitsui-Saito, M.; Amano, K.; Harada, K.; Miyamoto, S.; Nakazawa, H.; Won, K.J.; Sato, K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997, 49, 157–230. [Google Scholar]
- Jackson, W.F. Ion channels and vascular tone. Hypertension 2000, 35, 173–178. [Google Scholar] [CrossRef]
- McFadzean, I.; Gibson, A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Braz. J. Pharmacol. 2002, 135, 1–13. [Google Scholar] [CrossRef]
- Jin, L.; Teixeira, C.E.; Webb, R.C.; Leite, R. Comparison of the involvement of protein kinase C in agonist-induced contractions in mouse aorta and corpus cavernosum. Eur. J. Pharmacol. 2008, 590, 363–368. [Google Scholar] [CrossRef]
- Ma, J.; Pan, Z. Retrograde activation of store-operated calcium channel. Cell Calcium 2003, 33, 375–384. [Google Scholar] [CrossRef]
- Hayes, A.J.; Markovic, B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 2002, 40, 535–543. [Google Scholar] [CrossRef]
- Dijoux, N.; Guingand, Y.; Bourgeois, C.; Durand, S.; Fromageot, C.; Combe, C.; Ferret, P.J. Assessment of the phototoxic hazard of some essential oils using modified 3T3 neutral red uptake assay. Toxicol. In Vitro 2006, 20, 480–489. [Google Scholar] [CrossRef]
- Santoro, G.F.; Cardoso, M.G.; Guimarães, L.G.L.; Freire, J.M.; Soares, M.J. Anti-proliferative effect of the essential oil of Cymbopogon citratus (DC) Stapf (lemongrass) on intracellular amastigotes, bloodstream trypomastigotes and culture epimastigotes of Trypanosoma cruzi (Protozoa: Kinetoplastida). Parasitology 2007, 134, 1649–1656. [Google Scholar]
- Mesa-Arango, A.C.; Montiel-Ramos, J.; Zapata, B.; Durán, C.; Betancur-Galvis, L.; Stashenko, E. Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: Composition, cytotoxicity and antifungal activity. Mem. Inst. Oswaldo Cruz 2009, 104, 878–884. [Google Scholar] [CrossRef]
- Zapata-Sudo, G.; Pereira, S.L.; Beiral, H.J.V.; Kümmerle, A.E.; Raimundo, J.M.; Antunes, F.; Sudo, R.T.; Barreiro, E.J.; Fraga, C.A.M. Pharmacological characterization of (3-thienylidene)-3,4-methylenedioxybenzoylhydrazide: A novel muscarinic agonist with antihypertensive profile. Am. J. Hypertens. 2010, 23, 135–141. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, L.; Li, Y.; Du, G. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vascul. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds EOPB and citral are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, S.; Marques, A.; Sudo, R.T.; Kaplan, M.A.; Zapata-Sudo, G. Vasodilator Activity of the Essential Oil from Aerial Parts of Pectis brevipedunculata and Its Main Constituent Citral in Rat Aorta. Molecules 2013, 18, 3072-3085. https://doi.org/10.3390/molecules18033072
Pereira S, Marques A, Sudo RT, Kaplan MA, Zapata-Sudo G. Vasodilator Activity of the Essential Oil from Aerial Parts of Pectis brevipedunculata and Its Main Constituent Citral in Rat Aorta. Molecules. 2013; 18(3):3072-3085. https://doi.org/10.3390/molecules18033072
Chicago/Turabian StylePereira, Sharlene, Andre Marques, Roberto Takashi Sudo, Maria Auxiliadora Kaplan, and Gisele Zapata-Sudo. 2013. "Vasodilator Activity of the Essential Oil from Aerial Parts of Pectis brevipedunculata and Its Main Constituent Citral in Rat Aorta" Molecules 18, no. 3: 3072-3085. https://doi.org/10.3390/molecules18033072