Sesquiterpenoids from the Herb of Leonurus japonicus
Abstract
:1. Introduction
2. Results and Discussion
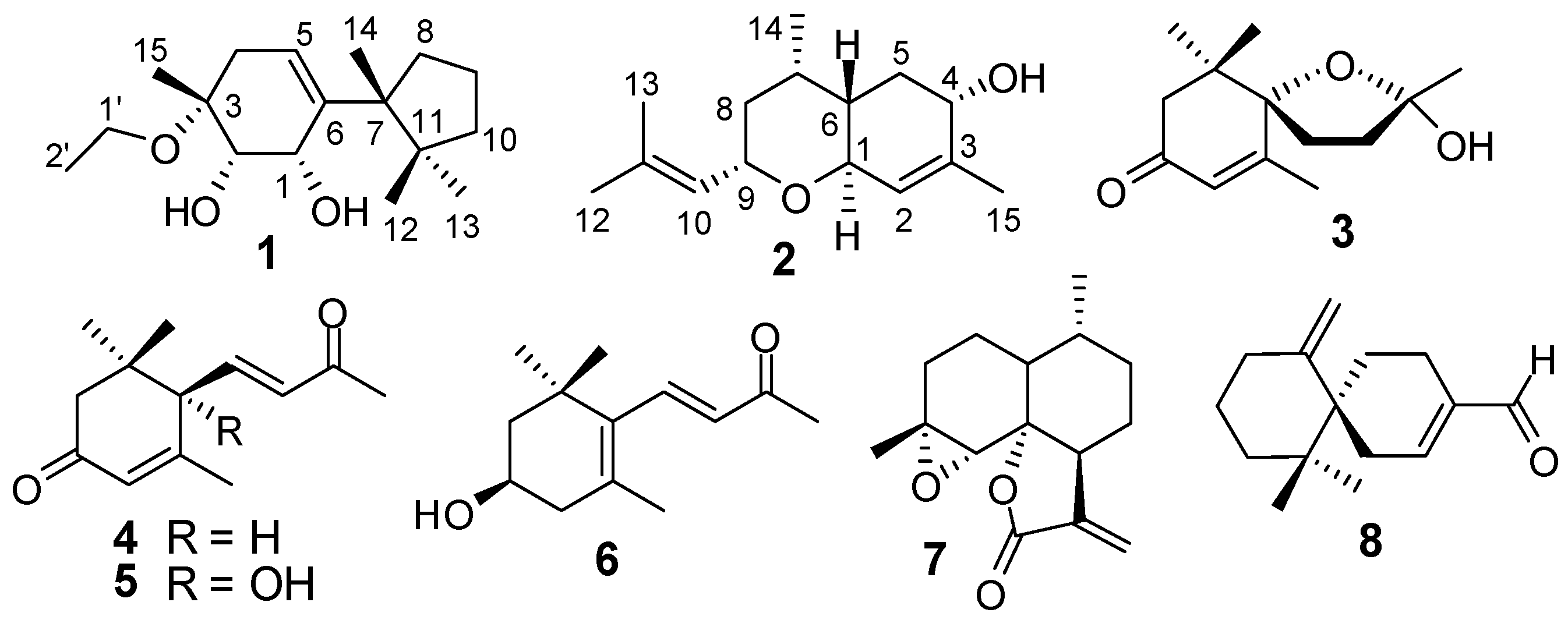
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 4.33 m | 70.1 | 4.25 br d (9.6) | 69.1 |
| 2 | 3.65 m | 74.9 | 5.38 br s | 127.1 |
| 3 | – | 76.1 | – | 139.3 |
| 4 | 2.30 dd (17.2, 4.0), 2.19 dd (17.2, 3.2) | 35.8 | 3.93 br d (4.8) | 66.8 |
| 5 | 5.62 dd (4.0, 3.2) | 123.1 | 2.16 dd (15.0, 3.6), 1.70 ddd (15.0, 4.8, 3.6) | 32.7 |
| 6 | – | 143.1 | 1.38 m | 39.5 |
| 7 | – | 50.8 | 2.24 m | 28.5 |
| 8 | 2.36 dd (8.4, 4.4), 1.71 dd (8.4, 3.2) | 36.9 | 1.42 ddd (12.6, 3.6, 3.0), 0.92 dd (12.6, 1.2) | 41.3 |
| 9 | 1.62 m | 19.7 | 4.22 m | 73.9 |
| 10 | 1.65 (overlapped), 1.44 dd (12.4, 4.0) | 40.4 | 5.11 d (7.8) | 128.4 |
| 11 | – | 45.9 | – | 133.8 |
| 12 | 0.82 s | 26.7 | 1.65 s | 18.4 |
| 13 | 1.00 s | 23.9 | 1.67 s | 25.7 |
| 14 | 1.06 s | 25.1 | 0.95 d (6.6) | 20.3 |
| 15 | 1.25 s | 19.7 | 1.79 s | 20.7 |
| 1′ | 3.49 m, 3.41 m | 56.8 | ||
| 2′ | 1.07 t (6.8) | 16.5 | ||
| OH-1 | 3.25 d (4.8) | – | ||
| OH-2 | 3.88 d (4.4) | – | ||
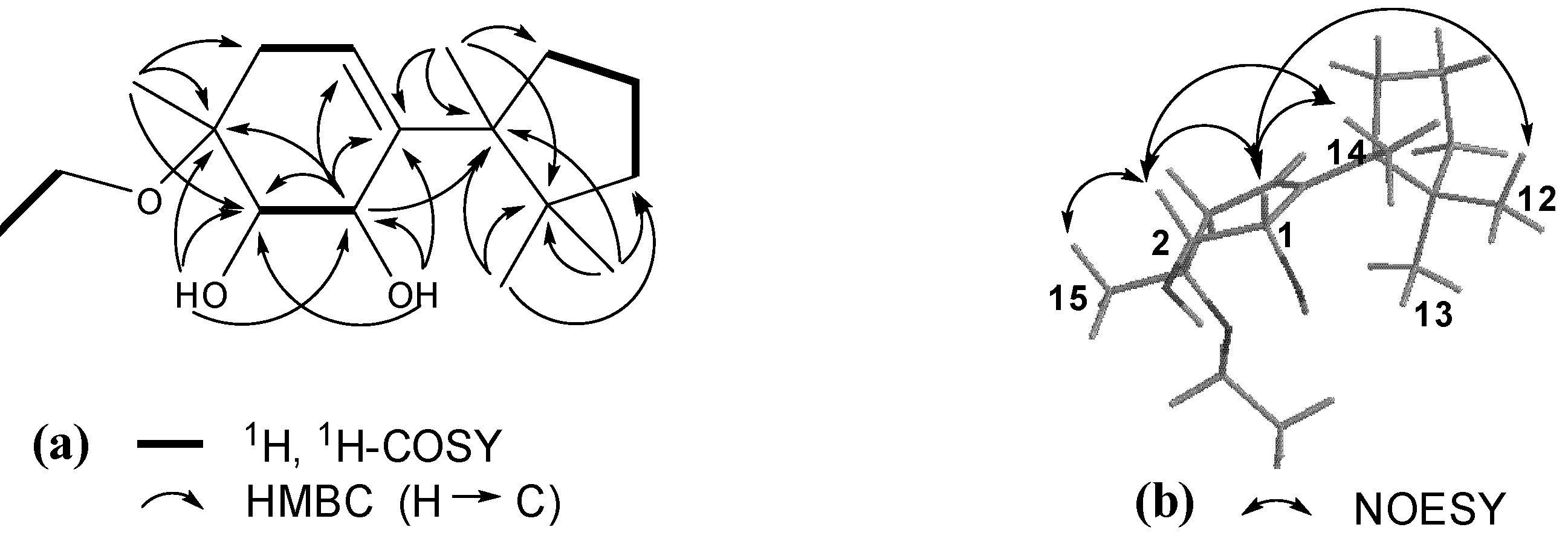
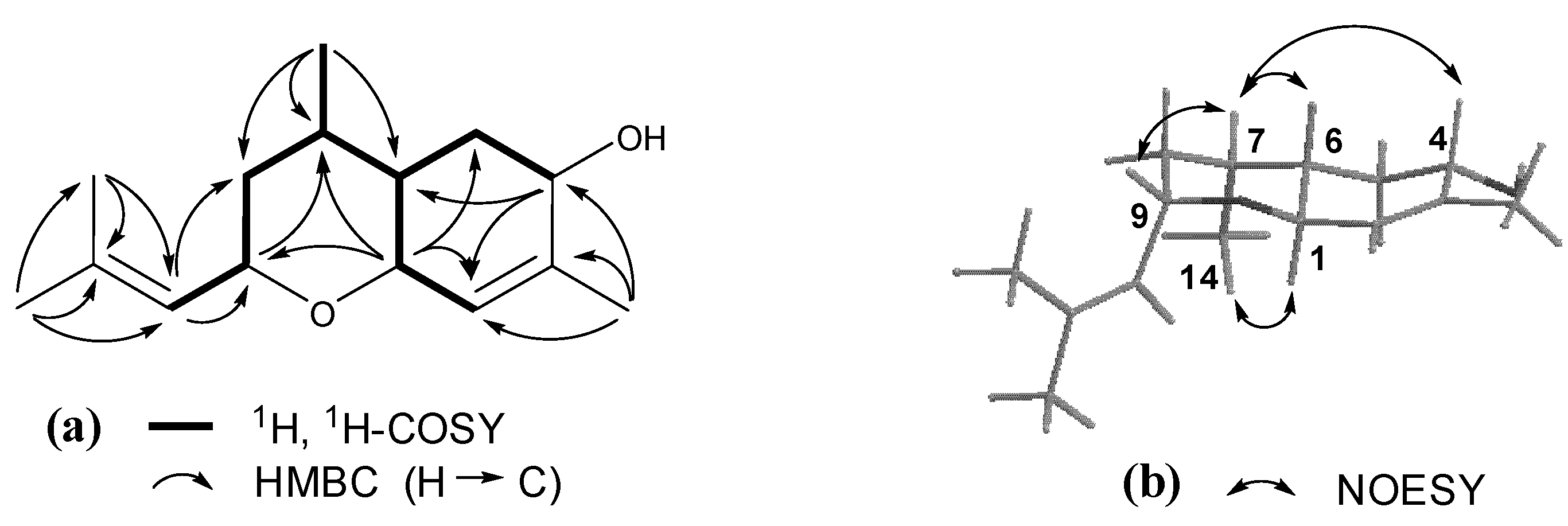
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
 = −5.0 (c = 0.10, MeOH); IR (KBr) νmax: 3,418, 3,039, 2,963, 2,928, 2,874, 1,462, 1,368, 1,237, 1,096, 1,057 cm−1; ESI-MS m/z 305.2 [M+Na]+; HRESI-MS: m/z 305.2090 [M+Na]+ (calcd for C17H30O3Na, 305.2093); 1H- and 13C-NMR data see Table 1.
= −5.0 (c = 0.10, MeOH); IR (KBr) νmax: 3,418, 3,039, 2,963, 2,928, 2,874, 1,462, 1,368, 1,237, 1,096, 1,057 cm−1; ESI-MS m/z 305.2 [M+Na]+; HRESI-MS: m/z 305.2090 [M+Na]+ (calcd for C17H30O3Na, 305.2093); 1H- and 13C-NMR data see Table 1. = −2.2 (c = 0.15, MeOH); IR (KBr) νmax: 3,478, 3,019, 2,925, 2,858, 1,461, 1,375, 1,230, 1,028 cm−1; ESI-MS m/z 259.2 [M+Na]+; HRESI-MS m/z 259.1668 [M+Na]+ (calcd for C15H24O2Na, 259.1674); 1H- and 13C-NMR data see Table 1.
= −2.2 (c = 0.15, MeOH); IR (KBr) νmax: 3,478, 3,019, 2,925, 2,858, 1,461, 1,375, 1,230, 1,028 cm−1; ESI-MS m/z 259.2 [M+Na]+; HRESI-MS m/z 259.1668 [M+Na]+ (calcd for C15H24O2Na, 259.1674); 1H- and 13C-NMR data see Table 1.3.4. Antibacterial Activity Experiments
3.5. Platelet Aggregation Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Li, Y.X.; Chen, Z.; Feng, Z.M.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Hepatoprotective glycosides from Leonurus japonicus Houtt. Carbohydr. Res. 2012, 348, 42–46. [Google Scholar] [CrossRef]
- Liu, X.H.; Pan, L.L.; Chen, P.F.; Zhu, Y.Z. Leonurine improves ischemia-induced myocardial injury through antioxidative activity. Phytomedicine 2010, 17, 753–759. [Google Scholar] [CrossRef]
- Qi, J.; Hong, Z.Y.; Xin, H.; Zhu, Y.Z. Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol. Pharm. Bull. 2010, 33, 1958–1964. [Google Scholar] [CrossRef]
- Tasdemir, D.; Scapozza, L.; Zerbe, O.; Linden, A.; Çalis, I.; Sticher, O. Iridoid glycosides of Leonurus persicus. J. Nat. Prod. 1999, 62, 811–816. [Google Scholar] [CrossRef]
- Morita, H.; Iizuka, T.; Gonda, A.; Itokawa, H.; Takeya, K. Cycloleonuripeptides E and F, cyclic nonapeptides from Leonurus heterophyllus. J. Nat. Prod. 2006, 69, 839–841. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, O.; Jin, H.G.; Woo, E.R.; Kang, S.S.; Baek, S.W.; Kim, J.; Kim, Y.S. Anti-inflammatory mechanism of 15,16-epoxy-3α-hydroxylabda-8,13(16),14-trien-7-one via inhibition of LPS-induced multicellular signaling pathways. J. Nat. Prod. 2012, 75, 67–71. [Google Scholar] [CrossRef]
- Boalino, D.M.; McLean, S.; Reynolds, W.F.; Tinto, W.F. Labdane diterpenes of Leonurus sibiricus. J. Nat. Prod. 2004, 67, 714–717. [Google Scholar] [CrossRef]
- Moon, H.T.; Jin, Q.; Shin, J.E.; Choi, E.J.; Han, H.K.; Kim, Y.S.; Woo, E.R. Bis-spirolabdane-type diterpenoids from Leonurus sibiricus. J. Nat. Prod. 2010, 73, 123–126. [Google Scholar]
- Gong, H.Q.; Wang, R.; Shi, Y.P. New labdane-type diterpenoids from Leonurus heterophyllus. Helv. Chim. Acta 2012, 95, 618–625. [Google Scholar] [CrossRef]
- Liu, Y.; Kubo, M.; Fukuyama, Y. Spirocyclic nortriterpenoids with NGF-potentiating activity from the fruits of Leonurus heterophyllus. J. Nat. Prod. 2012, 75, 1353–1358. [Google Scholar] [CrossRef]
- Commission of Chinese Pharmacopoeia, Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 272–273.
- Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Publishing House: Shanghai, China, 1995; pp. 1609−1610,1954–1956.
- Xiong, L.; Peng, C.; Zhou, Q.M.; Wan, F.; Xie, X.F.; Guo, L.; Li, X.H.; He, C.J.; Dai, O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules 2013, 18, 963–973. [Google Scholar] [CrossRef]
- Knapp, H.; Weigand, C.; Gloser, J.; Winterhalter, P. 2-Hydroxy-2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-en-8-one: precursor of 8,9-dehydrotheaspirone in white-fleshed nectarines. J. Agric. Food Chem. 1997, 45, 1309–1313. [Google Scholar] [CrossRef]
- Ma, M.; Bell, S.G.; Yang, W.; Hao, Y.; Rees, N.H.; Bartlam, M.; Zhou, W.; Wong, L.; Rao, Z. Structural analysis of CYP101C1 from Novosphingobium aromaticivorans DSM12444. ChemBioChem. 2011, 12, 88–89. [Google Scholar]
- Kai, H.; Baba, M.; Okuyama, T. Two new megastigmanes from the leaves of Cucumis sativus. Chem. Pharm. Bull. 2007, 55, 133–136. [Google Scholar] [CrossRef]
- DellaGreca, M.; Di Marino, C.; Zarrelli, A.; D’Abrosca, B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J. Nat. Prod. 2004, 67, 1492–1495. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Vishwakarma, R.A.; Jain, D.C.; Roy, R. High field NMR spectroscopic studies of arteannuin B and a reappraisal of the structure of arteannuin C. Phytochemistry 1991, 30, 3469–3471. [Google Scholar]
- Baek, N.I.; Han, J.T.; Ahn, E.M.; Park, J.K.; Cho, S.W.; Jeon, S.G.; Jang, J.S.; Kim, C.K.; Choi, S.Y. Isolation of anticonvulsant compounds from the fruits of Schizandra chinensis Baili. J. Korean Soc. Agric. Chem. Biotechnol. 2000, 43, 72–75. [Google Scholar]
- Nagashima, F.; Suzuki, M.; Takaoka, S.; Asakawa, Y. Sesqui- and diterpenoids from the Japanese liverwort Jungermannia infusca. J. Nat. Prod. 2001, 64, 1309–1317. [Google Scholar] [CrossRef]
- Warmers, U.; Rieck, A.; König, W.A.; Muhle, H. Bisabola-2,10-diene[1,9]oxide, a constituent of the liverwort Calypogeia suecica. Phytochemistry 1999, 51, 679–682. [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS), Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Sixth Edition: Approved Standard M7-A6; NCCLS: Wayne, PA, USA, 2003.
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiong, L.; Zhou, Q.-M.; Peng, C.; Xie, X.-F.; Guo, L.; Li, X.-H.; Liu, J.; Liu, Z.-H.; Dai, O. Sesquiterpenoids from the Herb of Leonurus japonicus. Molecules 2013, 18, 5051-5058. https://doi.org/10.3390/molecules18055051
Xiong L, Zhou Q-M, Peng C, Xie X-F, Guo L, Li X-H, Liu J, Liu Z-H, Dai O. Sesquiterpenoids from the Herb of Leonurus japonicus. Molecules. 2013; 18(5):5051-5058. https://doi.org/10.3390/molecules18055051
Chicago/Turabian StyleXiong, Liang, Qin-Mei Zhou, Cheng Peng, Xiao-Fang Xie, Li Guo, Xiao-Hong Li, Juan Liu, Zhao-Hua Liu, and Ou Dai. 2013. "Sesquiterpenoids from the Herb of Leonurus japonicus" Molecules 18, no. 5: 5051-5058. https://doi.org/10.3390/molecules18055051




