Volatile Aroma Compounds in Various Brewed Green Teas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Compounds in Prepared Green Teas
| Volatile Compounds | Odor characteristics | Reference |
|---|---|---|
| Aliphatic Alcohols | ||
| 1-Penten-3-ol | Butter, mild, green | [29] |
| 1-Pentanol | Somewhat sweet, balsamic | [29] |
| 2-Penten-1-ol | Jasmine, green, plastic, rubber | [29,30] |
| (Z)-3-Hexen-1-ol | ||
| Linalool | Bergamot oil, French lavender | [29] |
| Geraniol | Pleasant geranium | [29] |
| Aromatic Alcohols | ||
| Benzeneethanol | Floral, rose | [29] |
| 1-α-Terpineol | Lilac | [29] |
| 2-Methoxy-4-methylphenol | ||
| 2,6-Dimethyl-cyclohexanol | ||
| Aliphatic Aldehydes | ||
| 3-Methyl-butanal | Unpleasant | [29] |
| Pentanal | Pungent, almond, malt | [29,30] |
| Hexanal | Fruity | [29] |
| cis-3-Hexenal | herbal apple, green leafy | [29] |
| Nonanal | Fatty, citrus, green | [29,30] |
| 1H-Pyrrole-2-carboxaldehyde | ||
| Aromatic Aldehydes | ||
| Benzaldehyde | Bitter almond | [29] |
| Benzeneacetaldehyde | Hyacinth, lilac | [29] |
| Other Aromatic Compounds | ||
| Toluene | Paint | [30] |
| 1,4-Dimethoxybenzene | Aromatic ether, sweet clover | [29] |
| 1,4-Bis(1,1-dimethylethyl)-benzene | ||
| Styrene | Penetrating , balsamic, gasoline | [29,30] |
| 2-Hydroxy methyl ester benzoic acid | ||
| Ketones | ||
| 3,5-Octadien-2-one | ||
| Jasmone | Jasmine | [29] |
| α-Ionone | Woody, violet | [29] |
| β-Ionone | Woody | [29] |
| 2-Methyl-5-(1-methylethenyl)-2-cyclohexen-1-one (=Carvone) | Mint | [30,29] |
| Furans | ||
| Tetrahydro-2,2,5,5-tetramethyl furan | ||
| 4-Methyl-2-propyl furan | ||
| N-Furfuryl adenine | ||
| Pyridine | ||
| 3-Butyl-1-oxide-pyridine | ||
| Pyrazine | ||
| 3-Ethyl-2,5-dimethyl-pyrazine | ||
| Furanone | ||
| 5,6,7,7a-Tetra 2(4)-benzofuranone | ||
| Acids | ||
| Benzoic acid | Aromatic acid | |
| 2-Hydroxy methyl ester benzoic acid | ||
| Nonanoic acid | Fatty, green | [29,30] |
| Ester | ||
| Isopropyl myristate | Odorless | [29] |
2.1.1. Volatile Concentration
| Sample | 1-Penten -3-ol | 2-Penten -1-ol | Linalool | Geraniol | Benzeneethanol | 2,6-Di-methyl-cyclo- hexanol | Pentanal | Hexanal | Nonanal | Benz aldehyde | Toluene | 2-Hydroxy-3-methyl benzoic acid | Styrene | Jasmone | β-Ionone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kovats Retention Index | 760 | 859 | 1232 | 1420 | 1261 | 1259 | 780 | 889 | 1238 | 1281 | 1359 | 1006 | 1589 | 1691 | |
| Africa | |||||||||||||||
| Kenya 1 | 0.56 | 1.07 | 1.35 | - | - | - | 0.76 | - | 1.32 | 0.45 | - | - | - | - | - |
| Kenya 2 | 0.22 | 0.52 | 3.21 | 0.34 | - | - | - | 0.61 | - | 0.51 | - | - | - | - | 0.31 |
| Tanzania | - c | - | 1.56 | - | - | - | - | 0.44 | - | 0.54 | - | - | - | - | - |
| Southeast Asia and Indian Subcontinent | |||||||||||||||
| India | - | - | 0.96 | - | - | - | - | - | 0.77 | - | - | - | - | - | - |
| Sri Lanka 1 | - | 0.65 | 2.35 | - | - | - | 0.39 | 0.96 | 0.32 | - | - | 0.72 | - | - | 0.61 |
| Sri Lanka 2 | - | - | 0.83 | - | - | - | - | - | 1.17 | - | - | - | - | - | - |
| Vietnam | 0.80 | 0.64 | 0.74 | - | - | 0.33 | - | 0.61 | - | 0.74 | - | - | - | - | 0.80 |
| Northeast Asia | |||||||||||||||
| China 1 | - | - | - | - | - | - | - | - | 0.38 | - | - | 0.43 | - | - | - |
| China 2 | 0.14 | - | - | - | - | 0.48 | - | 0.37 | 0.22 | - | - | - | - | - | 0.62 |
| China 3 | - | - | 0.45 | 0.94 | - | - | - | 0.30 | 0.38 | - | 0.42 | - | - | 0.31 | 0.22 |
| China 4 | - | - | 1.34 | - | - | - | - | 0.28 | - | - | - | 0.52 | - | - | - |
| China 5 | - | - | 0.58 | 0.43 | 0.88 | - | - | 0.53 | 0.57 | 0.36 | 3.04 | - | - | 0.66 | - |
| China 6 | 0.29 | 1.52 | 0.45 | - | - | - | 0.92 | 0.73 | 0.61 | 0.65 | - | - | - | - | - |
| China 7 | - | - | 0.55 | 0.42 | 0.42 | - | - | 0.53 | 0.49 | 0.41 | - | - | - | 0.50 | - |
| Japan 1 | - | - | 0.50 | - | - | 0.66 | - | 0.54 | 0.48 | - | 0.86 | - | 0.25 | 1.21 | |
| Japan 2 | - | - | 0.27 | - | - | - | - | - | 0.41 | - | 0.35 | - | 0.21 | - | - |
| Japan 3 | - | - | 0.27 | 0.23 | 1.18 | - | 0.22 | 0.29 | 0.36 | 0.40 | 3.40 | - | - | 1.13 | 0.25 |
| Japan 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Japan 5 | - | - | 0.37 | - | - | 0.50 | 0.52 | 0.45 | - | - | - | - | 0.22 | - | 0.97 |
| Japan 6 | - | 0.48 | - | - | - | - | - | 0.36 | 0.34 | - | - | 0.55 | - | - | 0.53 |
| Korea 1 | - | - | 0.32 | - | 0.54 | - | - | 0.45 | - | - | - | - | - | - | - |
| Korea 2 | - | - | 0.39 | - | - | 0.40 | 0.31 | 0.43 | 0.37 | 0.27 | - | - | 0.18 | 0.52 | - |
| Korea 3 | 0.32 | 1.07 | 2.34 | 0.84 | 0.54 | - | - | 0.70 | 0.47 | 0.84 | - | - | - | 0.55 | 0.76 |
| Korea 4 | - | 0.98 | 0.60 | 0.58 | - | - | 0.96 | 0.62 | 0.33 | 0.70 | - | - | 1.06 | - | |
| Sample | 1-Pentanol | (Z)-3-Hexen-1-ol | 1-α-Terpineol | 2-Methoxy-4-methylphenol | 3-Methyl-butanal | 2,6-Dimethyl-cyclo hexanol | cis -3-Hexenal | 1H-Pyrrole-2-carboxaldehyde | Benzene-acetaldehyde | 1,4-Dimethoxy benzene | 1,4-Bis(1,1-dimethylethyl)-benzene | 3,5-Octadien-2-one |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | ||||||||||||
| Kenya 1 | ||||||||||||
| Kenya 2 | 0.21 | 2.57 | 1.21 | |||||||||
| Tanzania | 0.48 | |||||||||||
| Southeast Asia and Indian Subcontinent | ||||||||||||
| India | ||||||||||||
| Sri Lanka 1 | 0.45 | |||||||||||
| Sri Lanka 2 | ||||||||||||
| Vietnam | 1.0 | 0.35 | ||||||||||
| Northeast Asia | ||||||||||||
| China 1 | ||||||||||||
| China 2 | ||||||||||||
| China 3 | ||||||||||||
| China 4 | 0.27 | |||||||||||
| China 5 | 0.40 | |||||||||||
| China 6 | ||||||||||||
| China 7 | 0.33 | |||||||||||
| Japan 1 | ||||||||||||
| Japan 2 | ||||||||||||
| Japan 3 | ||||||||||||
| Japan 4 | ||||||||||||
| Japan 5 | ||||||||||||
| Japan 6 | ||||||||||||
| Korea 1 | ||||||||||||
| Korea 2 | 0.56 | 0.58 | ||||||||||
| Korea 3 | 0.33 | 5.34 | 2.69 | 0.29 | ||||||||
| Korea 4 | 0.27 | 0.47 | ||||||||||
| Africa | ||||||||||||
| Kenya 1 | ||||||||||||
| Kenya 2 | 0.93 | |||||||||||
| Tanzania | 0.67 | |||||||||||
| Southeast Asia and Indian Subcontinent | ||||||||||||
| India | ||||||||||||
| Sri Lanka 1 | 0.60 | 11.58 | ||||||||||
| Sri Lanka 2 | ||||||||||||
| Vietnam | ||||||||||||
| Northeast Asia | ||||||||||||
| China 1 | 0.53 | |||||||||||
| China 2 | 0.23 | 0.22 | ||||||||||
| China 3 | 0.29 | |||||||||||
| China 4 | 0.23 | |||||||||||
| China 5 | 0.30 | |||||||||||
| China 6 | ||||||||||||
| China 7 | ||||||||||||
| Japan 1 | 0.38 | 0.43 | ||||||||||
| Japan 2 | ||||||||||||
| Japan 3 | 0.18 | |||||||||||
| Japan 4 | 0.35 | |||||||||||
| Japan 5 | ||||||||||||
| Japan 6 | 1.21 | |||||||||||
| Korea 1 | ||||||||||||
| Korea 2 | ||||||||||||
| Korea 3 | 0.46 | 1.94 | ||||||||||
| Korea 4 | 0.26 | 0.85 | ||||||||||
2.1.2. Compounds Present
2.1.3. Composition Based on Geographic Area
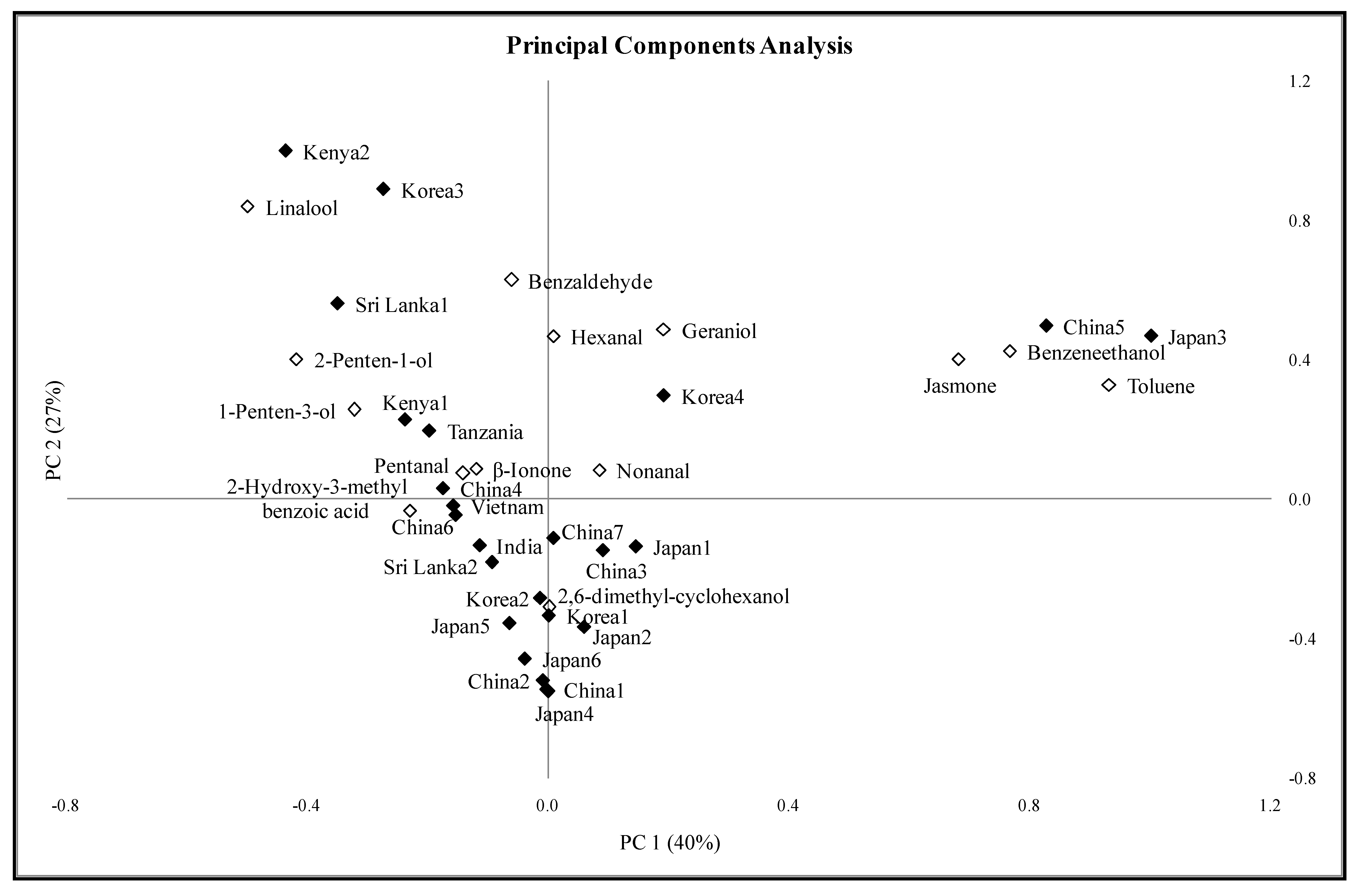
2.2. Principal Components Analysis of the Green Tea Samples
3. Experimental
3.1. Tea Samples
| Label a | Product name | Purchased from | |
|---|---|---|---|
| 1 | China 1 | Star of China | Imperialteagarden.com |
| 2 | China 2 | Gosancha | XingHua Food Co., Ltd |
| 3 | China 3 | Imperial Hwangshan Maofeng | Enjoyingtea.com |
| 4 | China 4 | Buddha’s Eyebrow | Sevencups.com |
| 5 | China 5 | Myeng Ding Sweet Dew | Sevencups.com |
| 6 | China 6 | Shanghai Special Gun Powder | Shanghai Tiantan Int'l trading Co., Ltd. |
| 7 | China 7 | Longjing (Dragonwell) | Adagio.com |
| 8 | India | Assam Sewpur Estate | Simpson & Vail Inc. (svtea.com) |
| 9 | Japan 1 | Tencha | Harney & Sons (Harney.com) |
| 10 | Japan 2 | Honyama Sencha | The Fragrant Leaf (thefragrantleaf.com) |
| 11 | Japan 3 | Sencha Overture | Adagio.com |
| 12 | Japan 4 | Uji Mecha | Itoen.com |
| 13 | Japan 5 | Uji Gyokuro | Japanesegreenteaonline.com |
| 14 | Japan 6 | Inakacha | Itoen.com |
| 15 | Kenya 1 | Kapchorua Green | Culinaryteas.com |
| 16 | Kenya 2 | Kapchorua Green | Barkingside.com |
| 17 | Korea 1 | Ssanggye Okchun | Lotte Department Store, Busan, Korea |
| 18 | Korea 2 | Sulloc Sejac | Donated by Amorepacific Co. |
| 19 | Korea 3 | Chungmyungcha | Lotte Department Store, Busan, Korea |
| 20 | Korea 4 | Tobu Goku (Guyu) | Tobu tea farm, Sunchon, Korea |
| 21 | Sri Lanka 1 | Iddalgashinna Estate Ceylon | Uptontea.com |
| 22 | Sri Lanka 2 | Dumbara Curls | Barkingside.com |
| 23 | Tanzania | Tanzania Luponde Estate | Simpson & Vail Inc. (svtea.com) |
| 24 | Vietnam | Ha Giang Green Tea | Thompsons (fineteas.com) |
3.2. Sample Preparation
3.3. Solid-Phase Microextraction
3.4. Gas Chromatograph-Mass Spectrometry
3.5. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ukers, W.H. All about Tea; The Tea and Coffee Trade Journal Company: New York, NY, USA, 1935; Volume 1, p. 568. [Google Scholar]
- Smyth, H.E.; Sanderson, J.E.; Sultanbawa, Y. Lexicon for the sensory description of Australian native plant foods and ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
- Fanaro, G.B.; Duarte, R.C.; Araujo, M.M.; Purgatto, E.; Villavicencio, A.L.C.H. Evaluation of gamma-radiation on green tea odor volatiles. Radiat. Phys. Chem. 2011, 80, 85–88. [Google Scholar] [CrossRef]
- Yamanishi, T. Flavour of green tea. Jpn. Agric. Res. Q. 1978, 12, 205–210. [Google Scholar]
- Kawakami, M.; Yamanishi, T. Aroma characteristics of Kabusecha (shaded green tea). J. Agric. Chem. Soc. Japan 1981, 55, 117–123. [Google Scholar]
- Cho, C.H. Changes of some compounds by heat treatment of green tea. M.S. Thesis, Ajou University, Suwon, Korea, 1983. [Google Scholar]
- Choi, S.H. Studies on flavor components of commercial Korean green tea. Korean J. Food Sci. Technol. 1991, 23, 98–101. [Google Scholar]
- Choi, S.H. The aroma components of commercial green tea picked in August. Korean J. Life Sci. 1995, 5, 20–24. [Google Scholar]
- Shimoda, M.; Shigematsu, H.; Shiratsuchi, H.; Osajima, Y. Comparison of the odor concentrates by SDE and adsorptive column method from green tea infusion. J. Agric. Food Chem. 1995, 43, 1616–1620. [Google Scholar] [CrossRef]
- Shimoda, M.; Shigematsu, H.; Shiratsuchi, H.; Osajima, Y. Comparison of volatile compounds among different grades of green tea and their relations to odor attributes. J. Agric. Food Chem. 1995, 43, 1621–1625. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Identification of potent odorants in different green tea varieties using flavor dilution technique. J. Agric. Food Chem. 2002, 50, 5660–5663. [Google Scholar] [CrossRef]
- Hattori, S.; Takagaki, H.; Fujimori, T. A comparison of the volatile compounds in several green teas. Food Sci. Technol. Res. 2005, 11, 82–86. [Google Scholar] [CrossRef]
- Kim, J.T. Science and Culture of Tea; Borim-sa: Seoul, Korea, 1996; p. 360. [Google Scholar]
- Ellis, H. Tea: Discovering, Exploring, Enjoying; Ryland Peters & Small: New York, NY, USA, 2002; p. 63. [Google Scholar]
- Lee, J.; Chambers, D.H. A lexicon for flavor descriptive analysis of green tea. J. Sens. Stud. 2007, 22, 256–272. [Google Scholar] [CrossRef]
- Jung, D.H. Components and Effects of Tea; Hongikjae: Seoul, South Korea, 2004; p. 456. [Google Scholar]
- Ryu, K.H.; Lee, H.J.; Park, S.K. Studies on the effect of low winter temperatures and harvest times on the volatile aroma compounds in green teas. Korean J. Food Sci. Technol. 2012, 44, 383–389. [Google Scholar] [CrossRef]
- Jumtee, K.; Komura, H.; Bamba, T.; Fukusaki, E. Predication of Japanese green tea (Sencha) ranking by volatile profiling using gas chromatography mass spectrometry and multivariate analysis. J. Biosci. Bioeng. 2011, 112, 252–255. [Google Scholar] [CrossRef]
- Hara, Y.; Luo, S.J.; Wickremasinghe, R.L.; Yamanishi, T. Special issue on tea. Food Rev. Int. 1995, 11, 371–542. [Google Scholar] [CrossRef]
- Xu, N.; Chen, Z.M. Green Tea, Black Tea and Semi-Fermented Tea. In Tea, Bioactivity and Therapeutic Potential; Zhen, Y.S., Chen, Z.M., Cheng, S.J., Chen, M.L., Eds.; Taylor & Francis: New York, NY, USA, 2002; pp. 35–55. [Google Scholar]
- Yamanishi, T.; Shimojo, S.; Ukita, M.; Kawashima, K.; Nakatani, Y. Aroma of roasted green tea (Hoji-cha). Agric. Biol. Chem. 1973, 37, 2147–2153. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yamanishi, T. Flavor components in Vietnamese green tea and lotus tea. Agric. Biol. Chem. 1975, 39, 1263–1267. [Google Scholar] [CrossRef]
- Takei, Y.; Ishiwata, K.; Yamanishi, T. Aroma components characteristic of spring green tea. Agric. Biol. Chem. 1976, 40, 2151–2157. [Google Scholar] [CrossRef]
- Liang, Y.R.; Ye, Q.; Jin, J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and instrumental assessment of green tea sensory preference. Int. J. Food Prop. 2008, 11, 258–272. [Google Scholar] [CrossRef]
- Ye, N.; Xhang, L.; Gu, X. Discrimination of green teas from different geographical origins by using HS-SPME/GC-MS and pattern recognition methods. Food Anal. Method 2012, 4, 856–860. [Google Scholar]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013. in press. Available online: http://dx.doi.org/10.1016/j.foodres (accessed on 19 August 2013).
- Burgard, D.R.; Kuznicki, J.T. Chemometrics: Chemical and Sensory Data; CRC Press: Boston, MA, USA, 1990; p. 17. [Google Scholar]
- Chambers, E., IV; Koppel, K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules 2013, 18, 4887–4905. [Google Scholar]
- Ash, M.; Ash, I. Handbook of Flavors and Fragrances; Synapse Information Resources, Inc.: Endicott, NY, USA, 2006. [Google Scholar]
- Acree, T.; Arn, H. Flavornet and human odor space. Available online: http://www.flavornet.org/ (accessed on 28 March 2013).
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Deutsche Forschungsanstalt für Lebensmittelchemie and Institut für Lebensmittelchemie der Technischen Universitat Munchen: Garching, Germany, 1998; pp. 6–63. [Google Scholar]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Chemicals: Aroma chemicals; S. Arctander: Copenhagen, Denmark, 1969. [Google Scholar]
- Kawakami, M.; Yamanishi, T. Flavor constituents of Longjing tea. Agric. Biol. Chem. 1983, 47, 2077–2083. [Google Scholar] [CrossRef]
- Lee, J.G.; Kwon, Y.J.; Chang, H.J.; Kwag, J.J.; Kim, O.C.; Choi, Y.H. Volatile components of green tea (Camellia sinensis L. var. Yabukita) by purge and trap headspace sampler. Korean J. Food Nut. 1997, 10, 25–30. [Google Scholar]
- Baptista, J.A.B.; da P Tavares, J.F.; Carvalho, R.C.B. Comparative study and partial characterization of Azorean green tea polyphenols. J. Food Compos. Anal. 1999, 12, 273–287. [Google Scholar] [CrossRef]
- Wang, L.F.; Lee, J.Y.; Chung, J.O.; Baik, J.H.; So, S.; Park, S.K. Discrimination of teas with different degrees of fermentation by SPME-GC analysis of the characteristic volatile flavour compounds. Food Chem. 2008, 109, 196–206. [Google Scholar] [CrossRef]
- Zhu, M.; Li, E.; He, H. Determination of volatile chemical constitutes in tea by simultaneous distillation extraction, vacuum hydrodistillation and thermal desorption. Chromatographia 2008, 68, 603–610. [Google Scholar] [CrossRef]
- Bott, L.; Chambers, E., IV. Sensory characteristics of combinations of chemicals potentially associated with beany aroma in foods. J. Sens. Stud. 2006, 21, 308–321. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shibamoto, T. Volatile constituents of green tea, Gyokuro (Camellia sinensis L. var Yabukita). J. Agric. Food Chem. 1981, 29, 366–370. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative activities of volatile extracts from green tea, oolong tea, and black tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef]
- Horita, H. Off-flavor components of green tea during preservation. Jpn. Agric. Res. Q. 1987, 21, 192–197. [Google Scholar]
- Choi, S.H.; Shin, M.K.; Lee, Y.J. Volatile aroma components of green tea scented with Lotus (Nelumbo nucifera Gaertner) flower. Food Sci. Biotechnol. 2003, 12, 540–543. [Google Scholar]
- Sawai, Y.; Yamaguchi, Y.; Tanaka, J. Methyl anthranilate is the cause of cultivar-specific aroma in the Japanese tea cultivar ‘Sofu’. Jpn. Agric. Res. Q. 2004, 38, 271–274. [Google Scholar]
- Yenket, R.; Chambers, E., IV; Adhikari, K. A comparison of seven preference mapping techniques using four software programs. J. Sens. Stud. 2011, 26, 135–150. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, J.; Chambers, D.H.; Chambers, E., IV; Adhikari, K.; Yoon, Y. Volatile Aroma Compounds in Various Brewed Green Teas. Molecules 2013, 18, 10024-10041. https://doi.org/10.3390/molecules180810024
Lee J, Chambers DH, Chambers E IV, Adhikari K, Yoon Y. Volatile Aroma Compounds in Various Brewed Green Teas. Molecules. 2013; 18(8):10024-10041. https://doi.org/10.3390/molecules180810024
Chicago/Turabian StyleLee, Jeehyun, Delores H. Chambers, Edgar Chambers, IV, Koushik Adhikari, and Youngmo Yoon. 2013. "Volatile Aroma Compounds in Various Brewed Green Teas" Molecules 18, no. 8: 10024-10041. https://doi.org/10.3390/molecules180810024







