Synthesis of a Novel Carbocyclic Analog of Bredinin
Abstract
:1. Introduction
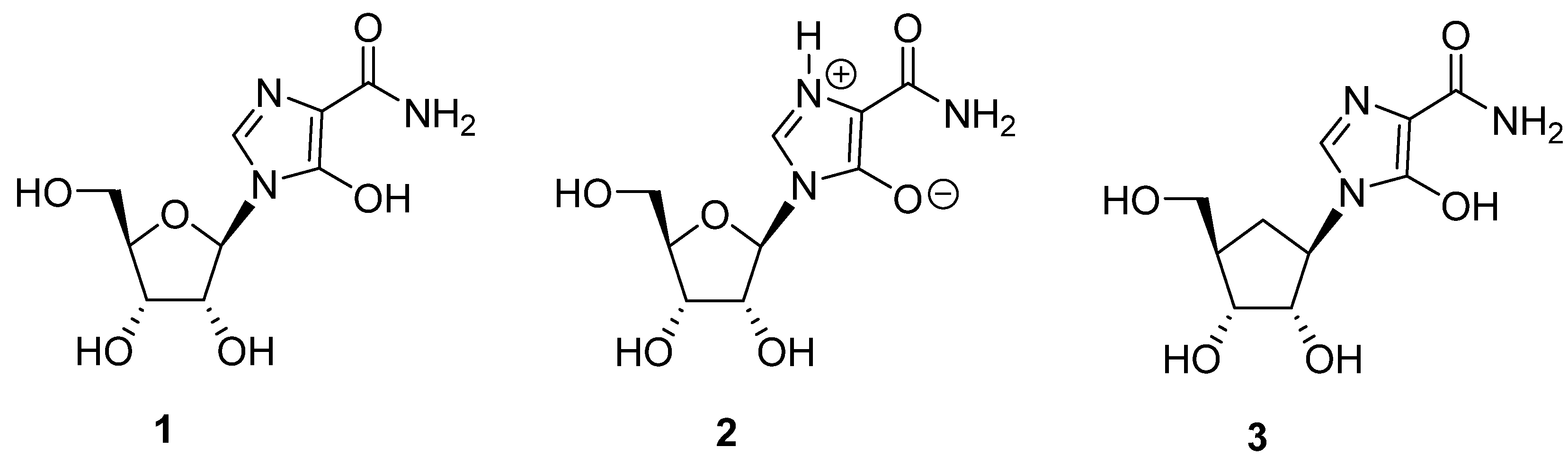
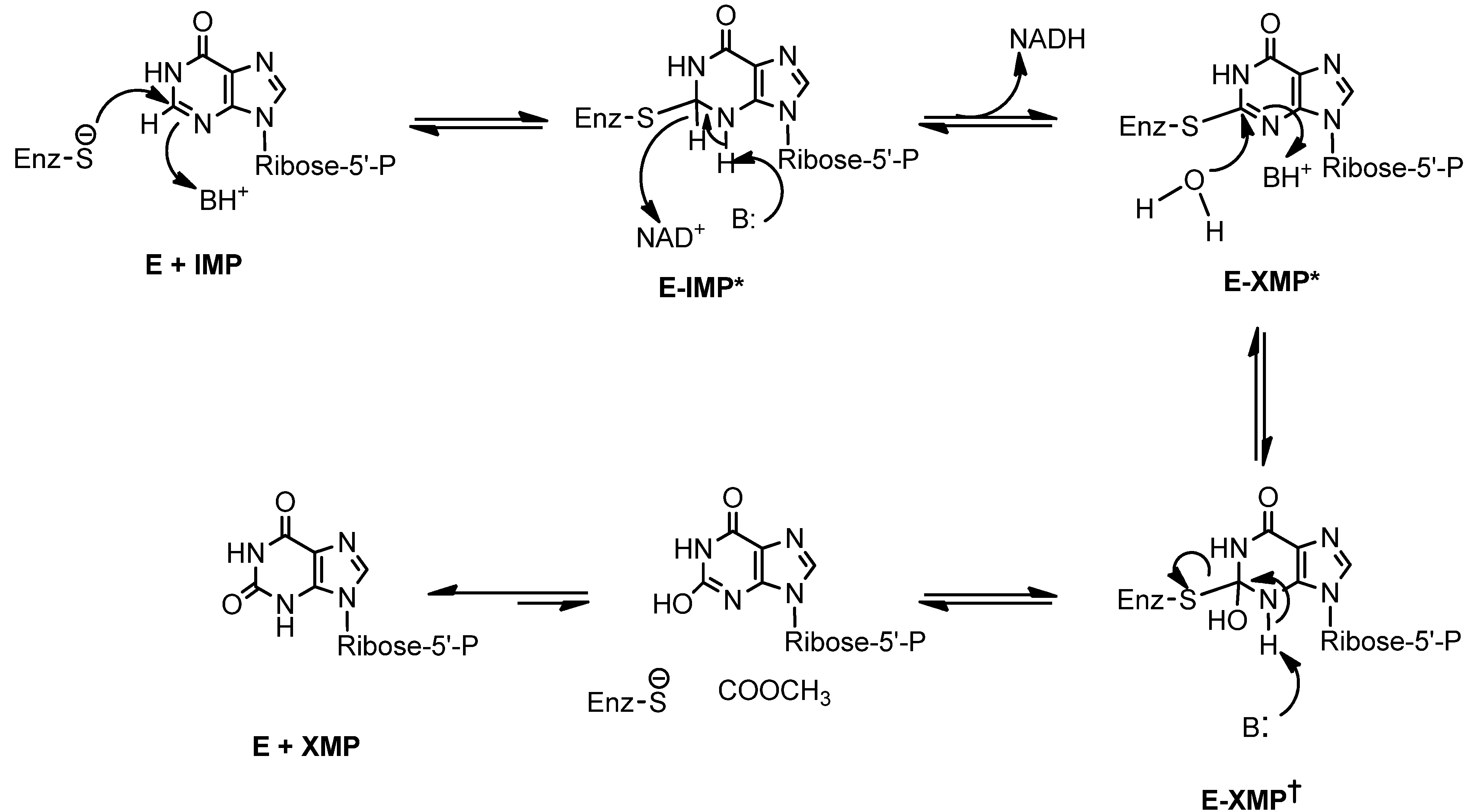
2. Results and Discussion
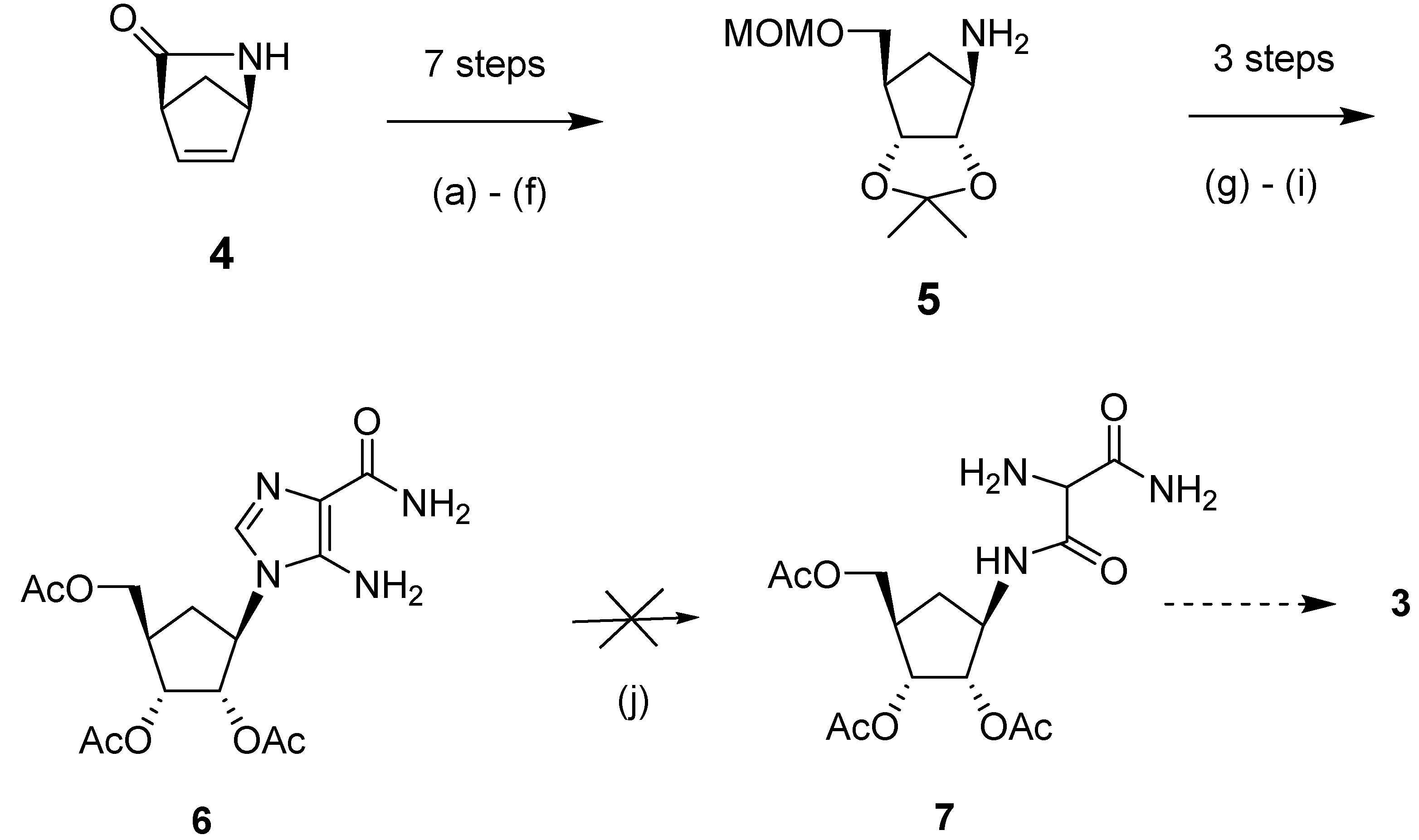
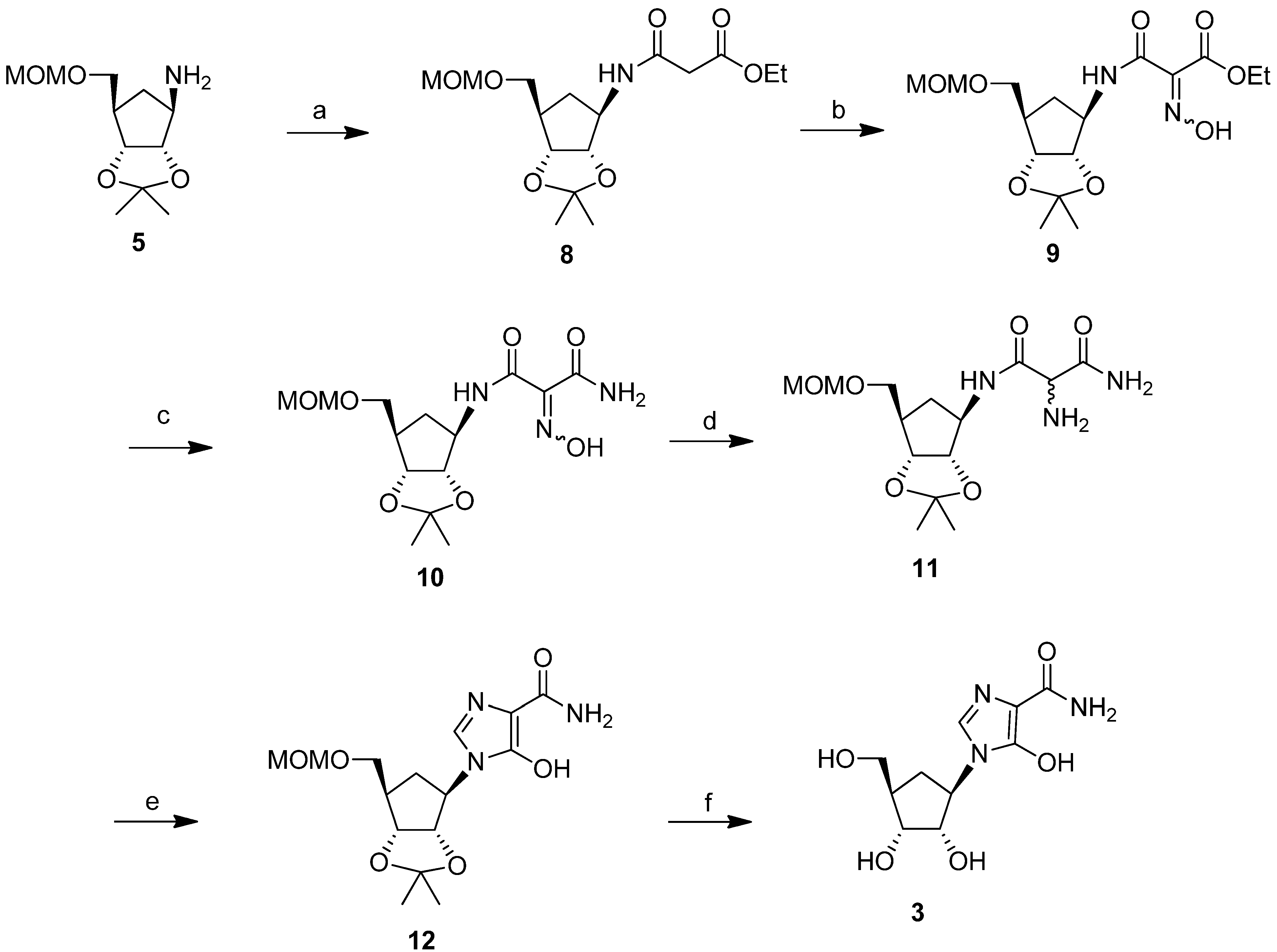
3. Experimental
3.1. General Procedures
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mizuno, K.; Tsujino, M.; Takada, M.; Hayashi, M.; Atsumi, K.; Asano, K.; Matsuda, T. Studies on bredinin.1. Isolation, characterization and biological properties. J. Antibiot. 1974, 27, 775–782. [Google Scholar] [CrossRef]
- Kosugi, Y.; Saito, Y.; Mori, S.; Watanabe, J.; Baba, M.; Shigeta, S. Antiviral activities of mizoribine and other inosine monophosphate dehydrogenase inhibitors against several ortho- and paramyxoviruses. Antivir. Chem. Chemoth. 1994, 5, 366–371. [Google Scholar]
- Yanagida, K.; Baba, C.; Baba, M. Inhibition of bovine viral diarrhea virus (BVDV) by mizoribine: synergistic effect of combination with interferon-α. Antivir. Res. 2004, 64, 195–201. [Google Scholar]
- Kato, N.; Naka, K.; Yano, M.; Abe, K.; Dansako, H.; Ikeda, M. Mizoribine: a new growth inhibitor for hepatitis C virus. Ther. Res. 2006, 27, 654–660. [Google Scholar]
- Naka, K.; Ikeda, M.; Abe, K.; Dansako, H.; Kato, N. Mizoribine inhibits hepatitis C virus RNA replication: Effect of combination with interferon-α. Biochem. Bioph. Res. Co. 2005, 330, 871–879. [Google Scholar] [CrossRef]
- Gan, L.; Seyedsayamdost, M.R.; Shuto, S.; Matsuda, A.; Petsko, G.A.; Hedstrom, L. The immunosuppressive agent mizoribine monophosphate forms a transition state analogue complex with inosine monophosphate dehydrogenase. Biochemistry 2003, 42, 857–863. [Google Scholar]
- Shiraki, K.; Ishibashi, M.; Okuno, T.; Kokado, Y.; Takahara, S.; Yamanishi, K.; Sonoda, T.; Takahashi, M. Effects of cyclosporine, azathioprine, mizoribine, and prednisolone on replication of human cytomegalovirus. Transplant Proc. 1990, 22, 1682–1685. [Google Scholar]
- Ishikawa, H. Mizoribine and mycophenolate mofetil. Curr. Med. Chem. 1999, 6, 575–597. [Google Scholar]
- Amemiya, H.; Itoh, H. Mizoribine (Bredinin). Mode of action and effects on graft rejection. In Immunosuppressive Drugs: Developments in Anti-Rejection Therapy; Thomson, A.W., Starzl, T.E., Eds.; Edward Arnold: London, UK, 1994; pp. 161–176. [Google Scholar]
- Gruber, S.A. Locoregional immunosuppression of organ transplants. Immunol. Rev. 1992, 129, 5–30. [Google Scholar] [CrossRef]
- Takei, S. Mizoribine in the treatment of rheumatoid arthirtis and juvenile idiopathic arthritis. Pediatr. Int. 2002, 44, 205–209. [Google Scholar] [CrossRef]
- Kerr, K.M.; Hedstrom, L. The roles of conserved carboxylate residues in IMP dehydrogenase and identification of a transition state analog. Biochemistry 1997, 36, 13365–13373. [Google Scholar] [CrossRef]
- Shu, Q.N.; Nair, V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med. Res. Rev. 2008, 28, 219–232. [Google Scholar] [CrossRef]
- Fukukawa, K.; Shuto, S.; Hirano, T.; Ueda, T. Synthesis of bredinin from 5-aminoimidazole-4-carboxamide-ribofuranoside (Aica-riboside). Chem. Pharm. Bull. 1984, 32, 1644–1646. [Google Scholar] [CrossRef]
- Nair, V.; Ma, X.; Shu, G.; Zhang, F.; Uchil, V.; Cherukupalli, G.R. IMPDH as a biological probe for RNA antiviral drug discovery: Synthesis, enzymology, molecular docking, and antiviral activity of new ribonucleosides with surrogate bases. Nucleos. Nucleot. Nucl. 2007, 26, 651–654. [Google Scholar] [CrossRef]
- Nair, V.; Zhang, F.; Ma, X.H.; Bonsu, E. Base-functionalized carbocyclic nucleosides: Design, Synthesis, and mechanism of antiviral activity. Nucleos. Nucleot. Nucl. 2009, 28, 408–423. [Google Scholar] [CrossRef]
- Pal, S.; Bera, B.; Nair, V. Inhibition of inosine monophosphate dehydrogenase (IMPDH) by the antiviral compound, 2-vinylinosine monophosphate. Bioorgan. Med. Chem. 2002, 10, 3615–3618. [Google Scholar] [CrossRef]
- Story, S.; Gupta, M.; Bonsu, E.; Nair, V. Inhibitors of inosine monophosphate dehydrogenase: Probes for antiviral drug discovery. Nucleos. Nucleot. Nucl. 2005, 24, 717–720. [Google Scholar] [CrossRef]
- Nair, V.; Jahnke, T.S. Antiviral activities of isomeric dideoxynucleosides of D- and L-related stereochemistry. Antimicrob. Agents Ch. 1995, 39, 1017–1029. [Google Scholar] [CrossRef]
- Daluge, S.; Vince, R. Synthesis of carbocyclic aminonucleosides. J. Org. Chem. 1978, 43, 2311–2320. [Google Scholar] [CrossRef]
- Cermak, R.C.; Vince, R. 4-Beta-amino-2-alpha,3-alpha-dihydroxy-1-beta-cyclopentanemethanol hydrochloride-carbocyclic ribofuranosylamine for the synthesis of carbocyclic nucleosides. Tetrahedron Lett. 1981, 22, 2331–2332. [Google Scholar] [CrossRef]
- Taylor, S.J.C.; McCague, R.; Wisdom, R.; Lee, C.; Dickson, K.; Ruecroft, G.; O’Brien, F.; Littlechild, J.; Bevan, J.; Roberts, S.M.; et al. Development of the biocatalytic resolution of 2-azabicyclo[2.2.1]hept-5-en-3-one as an entry to single-enantiomer carbocyclic nucleosides. Tetrahedron-Asymmetr. 1993, 4, 1117–1128. [Google Scholar] [CrossRef]
- Hutchinson, E.J.; Taylor, B.F.; Blackburn, G.M. Synthesis of carbocyclic NAD(+) containing a methylenebisphosphonate linkage for the investigation of ADP-ribosyl cyclase. Chem. Commun. 1996, 2765–2766. [Google Scholar] [CrossRef]
- Fukukawa, K.; Shuto, S.; Hirano, T.; Ueda, T. Synthesis of bredinin from 1-beta-D-ribofuranosyl-5-aminoimidazole-4-carboxamide by a photo-reaction. Chem. Pharm. Bull. 1986, 34, 3653–3657. [Google Scholar] [CrossRef]
- Shuto, S.; Haramuishi, K.; Fukuoka, M.; Matsuda, A. Synthesis of sugar-modified analogs of bredinin (mizoribine), a clinically useful immunosuppressant, by a novel photochemical imidazole ring-cleavage reaction as the key step. J. Chem. Soc., Perkin Trans. 1 2000, 3603–3609. [Google Scholar]
- Humble, R.W.; Middleton, D.F.; Banoub, J.; Ewing, D.F.; Boa, A.N.; Mackenzie, G. A synthesis of bredinin (Mizoribine®) from an acyclic precursor. Tetrahedron Lett. 2011, 52, 6223–6227. [Google Scholar]
- Shuto, S.; Haramuishi, K.; Matsuda, A. Nucleosides and nucleotides .145. Synthesis of 2'-deoxy and 5'-phosphate derivatives of bredinin. A photochemical imidazole-ring cleavage and subsequent reconstruction of the base moiety. Tetrahedron Lett. 1996, 37, 187–190. [Google Scholar] [CrossRef]
- Nair, V.; Chi, G.; Shu, Q.; Julander, J.; Smee, D.F. A heterocyclic molecule with significant activity against dengue virus. Bioorgan. Med. Chem. Lett. 2009, 19, 1425–1427. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3, 6, 8, 9, 10, 11, and 12 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nair, V.; Zhang, F. Synthesis of a Novel Carbocyclic Analog of Bredinin. Molecules 2013, 18, 11576-11585. https://doi.org/10.3390/molecules180911576
Nair V, Zhang F. Synthesis of a Novel Carbocyclic Analog of Bredinin. Molecules. 2013; 18(9):11576-11585. https://doi.org/10.3390/molecules180911576
Chicago/Turabian StyleNair, Vasu, and Fan Zhang. 2013. "Synthesis of a Novel Carbocyclic Analog of Bredinin" Molecules 18, no. 9: 11576-11585. https://doi.org/10.3390/molecules180911576



