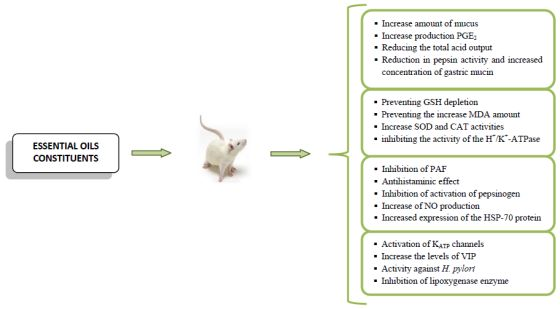
Anti-Ulcer Activity of Essential Oil Constituents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Menthol
2.2. Isopulegol
2.3. Limonene
2.4. Cineole
2.5. Thymoquinone
2.6. Carvacrol
2.7. α-Terpineol
2.8. Terpinen-4-ol
2.9. Epoxycarvone
2.10. Elemol
2.11. Nerolidol
2.12. α-Bisabolol
2.13. Anethole
2.14. Eugenol
2.15. 1′S-1′-Acetoxychavicol and 1′S-1′-Acetoxyeugenol Acetate
2.16. Cinnamaldehyde
2.17. Cinnamic Acid
2.18. Citral
2.19. Thymol
2.20. Bisabolangelone
3. Conclusions
Supplementary Materials
Abbreviations
| c-AMP | Cyclic adenosine monophosphate |
| CAT | Catalase |
| COX-2 | Cyclo-oxygenase-2 |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GSH | Glutathione |
| GSH-px | Glutathione peroxidase |
| H+/K+-ATPase | Hydrogen potassium ATPase |
| HCl | Hydrochloric acid |
| HSP-70 | Heat shock protein 70 |
| IC | Inhibitory concentration |
| KATP channels | ATP-sensitive potassium channel |
| L-NAME | N (G)-nitro-L-arginine methyl ester |
| LTB4 | Leukotriene B4 |
| LTC4 | Leukotriene C4 |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| NDGA | Nordihydroguaiaretic acid |
| NO | nitric oxide |
| NOS | Nitric oxide synthase |
| NP-SH | Nonprotein sulfhydryls |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PAF | Platelet-activating factor |
| PGE2 | Prostaglandin E2 |
| pH | Potential of hydrogen |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factoralpha |
| VIP | Vasoactive intestinal peptide |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zapata-Colindres, J.C.; Zepeda-Gómez, S.; Montaño-Loza, A.; Vázquez-Ballesteros, E.; de Jesús Villalobos, J.; Valdovinos-Andraca, F. The association of Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs in peptic ulcer disease. Can. J. Gastroenterol. 2006, 20, 277–280. [Google Scholar]
- Marcus, E.A.; Vagin, O.; Tokhtaeva, E.; Sachs, G.; Scott, D.R. Helicobacter pylori impedes acid-induced tightening of gastric epithelial junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G731–G739. [Google Scholar] [CrossRef]
- Prabhu, V.; Shivani, A. An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann. Med. Health Sci. Res. 2014, 4, 22–29. [Google Scholar] [CrossRef]
- Klein, L.C., Jr.; Gandolfi, R.B.; Santin, J.R.; Lemos, M.; Cechinel-Filho, V.; Andrade, S.F. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Poligalaceae). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 381, 121–126. [Google Scholar] [CrossRef]
- Behrman, S.W. Management of complicated peptic ulcer disease. Arch. Surg. 2005, 140, 201–208. [Google Scholar] [CrossRef]
- Lockrey, G.; Lima, L. Peptic ulcer disease in older people. J. Pharm. Pract. Res. 2011, 41, 58–61. [Google Scholar]
- Gadekar, R.; Singour, P.K.; Chaurasiya, P.K.; Pawar, R.S.; Patil, U.K. A potential of some medicinal plants as an antiulcer agents. Pharmacogn. Rev. 2010, 4, 136–146. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Chan, F.K.; Mccoll, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Awaad, A.S.; El-Meligy, R.M.; Soliman, G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013, 17, 101–124. [Google Scholar] [CrossRef]
- Lakshimi, V.; Singh, N.; Shrivastva, S.; Mishra, S.K.; Dharmani, P.; Palit, G. Gedunin and photogedunin of Xylocarpus granatum show significant anti-secretory effects and protect the gastric mucosa of peptic ulcer in rats. Phytomedicine 2009, 17, 569–574. [Google Scholar]
- Sheen, E.; Triadafilopoulos, G. Adverse effects of long-term proton pump inhibitor therapy. Dig. Dis. Sci. 2011, 56, 931–950. [Google Scholar] [CrossRef]
- Zayachkivska, O.S.; Konturek, S.J.; Drozdowicz, D.; Brzozowski, T.; Gzhegotsky, M.R. Influence of plant-originated gastroprotective and antiulcer substances on gastric mucosal repair. Fiziol. Zh. 2004, 50, 118–127. [Google Scholar]
- Schmeda-Hirschmann, G.; Yesilada, E. Traditional medicine and gastroprotective crude drugs. J. Ethnopharmacol. 2005, 22, 61–66. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- De Sousa, D.P. Medicinal Essential Oils: Chemical, Pharmacological and Therapeutic Aspects, 1st ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 1–236. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hanson, P.J. Anti-ulcer drugs of plant origin. Prog. Med. Chem. 1991, 28, 201–231. [Google Scholar] [CrossRef]
- Rozza, A.L.; Hiruma-Lima, C.A.; Takahira, R.K.; Padovani, C.R.; Pellizzon, C.H. Effect of menthol in experimentally induced ulcers: Pathways of gastroprotection. Chem. Biol. Interact. 2013, 206, 272–278. [Google Scholar] [CrossRef]
- Shah, K.; Shrivastava, S.; Mishra, P. Evaluation of mefenamic acid mutual prodrugs. Med. Chem. Res. 2013, 22, 70–77. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Shanbhag, V.R.; Rider, A.M.; Gokhale, R.; Harpalanai, A.; Dick, R.M. Ester and amide prodrugs of ibuprofen and naproxen: Synthesis, anti-inflammatory activity and gastrointestinal toxicity. J. Pharm. Sci. 1992, 81, 149–154. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Akhter, M. Synthesis, pharmacological activity and hydrolytic behavior of glyceride prodrugs of ibuprofen. Eur. J. Med. Chem. 2005, 40, 371–376. [Google Scholar] [CrossRef]
- Redasani, V.K.; Sanjay, B.B. Synthesis and evaluation of mutual prodrugs of ibuprofen with menthol, thymol and eugenol. Eur. J. Med. Chem. 2012, 56, 134–138. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S.N. 1,8-Cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Digest. Dis. Sci. 2001, 46, 331–337. [Google Scholar] [CrossRef]
- Vernin, G.A.; Parkanyi, C.; Cozzolino, F.; Fellous, R. GC/MS analysis of the volatile constituents of Corymbia citriodora Hook. from Réunion Island. J. Essent. Oil Res. 2004, 16, 560–565. [Google Scholar] [CrossRef]
- Paik, S.Y.; Kok, K.H.; Beak, S.M.; Paek, S.H.; Kim, J.A. The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol. Pharm. Bull. 2005, 28, 802–807. [Google Scholar] [CrossRef]
- Serra, S.; Brenna, E.; Fuganti, C.; Maggioni, F. Lipase-catalyzed resolution of p-menthan-3-ols monoterpenes: Preparation of the enantiomer-enriched forms of menthol, isopulegol, trans- and cis-piperitol, and cis-isopiperiten. Tetrahedron Asymmetry 2003, 14, 3313–3319. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on alpha-bisabolol. Food Chem. Toxicol. 2008, 46, 72–76. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on isopulegol. Food Chem. Toxicol. 2008, 46, 185–189. [Google Scholar] [CrossRef]
- Spindler, P.; Madsen, C. Subchronic toxicity study of peppermint oil in rats. Toxicol. Lett. 1992, 62, 215–220. [Google Scholar] [CrossRef]
- Silva, M.I.G.; Moura, B.A.; Neto, M.R.A.; Tomé, A.R.; Rocha, N.F.M.; Carvalho, A.M.R; Macêdo, D.S.; Vasconcelos, S.M.M.; Sousa, D.P.; Viana, G.S.B.; et al. Gastroprotective activity of isopulegol on experimentally induced gastric lesions in mice: Investigation of possible mechanisms of action. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 380, 233–245. [Google Scholar] [CrossRef]
- Robert, A. Cytoprotection by prostaglandins. Gastroenterology 1979, 77, 761–767. [Google Scholar]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Szabo, S. Mechanisms of Mucosal Injury in the Stomach and Duodenum: Time-sequence Analysis of Morphologic, Functional, Biochemical and Histochemical Studies Scandinavian. J. Gastroenterol. 1987, 22, 21–28. [Google Scholar]
- Nguemfo, E.L.; Dimo, T.; Dongmo, A.B.; Azebaze, A.G.; Alaoui, K.; Asongalem, A.E.; Cherrah, Y.; Kamtchouing, P. Anti-oxidative and anti-inflammatory activities of some isolated constituents from the stem bark of Allanblackia monticola Staner L.C (Guttiferae). Inflammopharmacology 2009, 17, 37–41. [Google Scholar] [CrossRef]
- Miller, T.A. Protective effects of prostaglandins against gastric mucosal damage: Current knowledge and proposed mechanisms. Am. J. Physiol. 1983, 245, 601–623. [Google Scholar]
- Rainsford, K.D. Structure-activity relationships of non-steroid anti-inflammatory drug gastric ulcerogenic activity. Agents Actions 1978, 8, 587–605. [Google Scholar] [CrossRef]
- Wallace, J.L. Nitric oxide, aspirin-triggered lipoxins and NO aspirin in gastric protection. Inflamm. Allergy Drug Targets 2006, 5, 133–137. [Google Scholar] [CrossRef]
- Peskar, B.M.; Ehrlich, K.; Peskar, B.M. Role of ATP-Sensitive Potassium Channels in Prostaglandin-Mediated Gastroprotection in the Rat. J. Pharmacol. Exp. Ther. 2002, 301, 969–974. [Google Scholar] [CrossRef]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef]
- Meral, G.E.; Konyalioglu, S.; Ozturk, B. Essential oil composition and antioxidant activity of endemic Ziziphora taurica subsp. cleonioides. Fitoter 2002, 73, 716–718. [Google Scholar] [CrossRef]
- Siani, A.C.; Garrido, I.S.; Monteiro, S.S.; Carvalho, E.S.; Ramos, M.F.S. Protium icicariba as a source of volatile essences. Biochem. Syst. Ecol. 2004, 32, 477–489. [Google Scholar] [CrossRef]
- Amaral, M.P.M.; Braga, F.A.V.; Passos, F.F.B.; Almeida, F.R.C.; Oliveira, R.C.M.; Carvalho, A.A.; Arruda, D.; Alexandri, F.D.; Katzin, A.; Uliana, S. Antileishmanial activity of the terpene Nerolidol. Clin. Exp. Pharmacol. Physiol. 2005, 49, 1679–1687. [Google Scholar]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Rocha, L.R.M.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Rozza, A.L.; Moraes, T.M.; Kushima, H.; Tanimoto, A.; Marques, M.O.M.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef]
- Baananou, S.; Bouftira, I.; Mahmoud, A.; Boukef, K.; Marongiu, B.; Boughattas, N.A. Antiulcerogenic and antibacterial activities of Apium graveolens essential oil and extract. Nat. Prod. Res. 2013, 27, 1075–1083. [Google Scholar] [CrossRef]
- Al Moutaery, A.R. Protective effect of ketoconazole against experimentally induced gastric ulcers in rats. Res. Commun. Mol. Pathol. Pharmacol. 2003, 113, 5–23. [Google Scholar]
- Tunçel, N.; Tunçel, M.; Aboul-Enein, H.Y. Effects of the vasoactive intestinal peptide on stress-induced mucosal ulcers and modulation of methylation of histamine in gastric tissue of the rats. Il Farmaco 2003, 58, 449–454. [Google Scholar] [CrossRef]
- Szabo, S.; Vattay, P. Experimental gastric and duodenal ulcers. Advances in pathogenesis. Gastroenterol. Clin. N. Am. 1990, 19, 67–85. [Google Scholar]
- Muhammad, J.S.; Sugiyama, T.; Zaidi, S.F. Gastric pathophysiological ins and outs of helicobacter pylori: A review. J. Pak. Med. Assoc. 2013, 63, 1528–1533. [Google Scholar]
- Alarcon, T.; Domingo, D.; Lopez-Brea, M. Antibiotic resistance problems with Helicobacter pylori. Int. J. Antimicrob. Agents 1999, 12, 19–26. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Drake, S.; Gollapudi, S.R.; Okwute, K. Model look at folkloric use of anti-infective agents. J. Nat. Prod. 1987, 50, 1025–1040. [Google Scholar] [CrossRef]
- Sanguinetti, E.E. Plantas Que Curam, 2nd ed.; Editora Rígel: Porto Alegre, RS, Brazil, 1989; p. 208. [Google Scholar]
- Bandhopadhyay, U.; Biswas, K.; Chatterjee, R.; Kumar Ganguly, I.C.C.; Bhattacharya, K.; Banerjee, R. Gastroprotective effect of Neem (Azadiracta indica) bark extract: Possible involvement of H+K+ATPase inhibition and scavenging of hydroxyl radical. Life Sci. 2002, 71, 2845–2865. [Google Scholar] [CrossRef]
- Martin, G.R.; Wallace, J.L. Gastrointestinal inflammation: A central component of mucosal defense and repair. Exp. Biol. Med. 2006, 231, 130–137. [Google Scholar]
- Rainsford, K.D. The effect of 5-lipoxygenase inhibitors and leukotriene antagonists on the development of gastric lesions induced by non steroidal anti-inflammatory drugs in mice. Agents Action 1987, 21, 316–319. [Google Scholar] [CrossRef]
- Glavin, G.B.; Szabo, S. Experimental gastric mucosal injury, laboratory models reveal mechanism of pathogenesis and new therapeutic strategies. FASEB J. 1992, 6, 825–831. [Google Scholar]
- Breggia, M.E.; Miguenz, M.; Silberman, P.E.; Laudisi, C.; Lemberg, A.; Filinger, E. Fármacos usados para el Control de la Acidez Gástrica y el Tratamiento de la Úlcera Péptica. Acta Farmacéut. Bonaer. 2009, 19, 133–142. (In Spanish) [Google Scholar]
- Flemstrom, G.; Isenberg, J.I. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol. Sci. 2001, 16, 23–28. [Google Scholar]
- Tariq, M.; Khan, H.A.; Elfaki, I.; Arshaduddin, M.; Al Moutaery, M.; Al Rayes, H.; Al Swailam, R. Gastric antisecretory and antiulcer effects of simvastatin in rats. J. Gastroenterol. Hepatol. 2007, 22, 2316–2323. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Silano, M.; de Vincenzi, A.; Maialetti, F.; Scazzocchio, B. Safety data review: Constituents of aromatic plants: Eucalyptol. Fitoterapia 2002, 73, 269–275. [Google Scholar] [CrossRef]
- Kirsch, F.; Beauchamp, J.; Buettner, A. Time-dependent aroma changes in breast milk after oral intake of a pharmacological preparation containing 1,8-cineole. J. Clin. Nutr. 2012, 31, 682–692. [Google Scholar] [CrossRef]
- Pattnaick, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39–46. [Google Scholar]
- Peskar, B.M.; Lange, K.; Hoppe, U.; Peskar, B.A. Ethanol stimulates formation of leukotriene C4 in ratgastric mucosa. Prostaglandins 1986, 31, 283–293. [Google Scholar]
- Alarcon, L.L.C.; Martin, M.J.; Marhuenda, E. Gastric anti-ulcer activity of silymarin, a lipoxygenase inhibitor, in rats. J. Pharm. Pharmacol. 1992, 44, 929–931. [Google Scholar] [CrossRef]
- Boughton-Smith, N.K.; Bhatia, S.P.; McGinty, D.; Letiziam, C.S.; Api, A.M. Involvement of leukotrienes in acute gastric damage. Methods Find. Exp. Clin. Pharmacol. 1989, 11, 53–59. [Google Scholar]
- Juergens, U.R.; Stöber, M.; Vetter, H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar]
- Santos, F.A.; Silva, R.M.; Campos, A.R.; de Araújo, R.P.; Lima Júnior, R.C.; Rao, V.S. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem. Toxicol. 2004, 42, 579–584. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, V.; Chaudhary, A.K. Gastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud. in Wistar rats. J. Ethnopharmacol. 2011, 134, 294–297. [Google Scholar] [CrossRef]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef]
- Kruk, I.; Michalska, T.; Lichszteld, K.; Køadna, A.; Aboul-Enein, H.Y. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 2000, 41, 1059–1064. [Google Scholar] [CrossRef]
- El-Abhar, H.S.; Abdallah, D.M.; Saleh, S. Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia/reperfusion in rats. J. Ethnopharmacol. 2003, 84, 251–258. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Ueda, S.; Ichikawa, H.; Takahashi, S.; Yasuda, M.; Kondo, M. Ischemia–reperfusion injury and free radical involvement in gastric mucosal disorders. Adv. Exp. Med. Biol. 1992, 316, 231–238. [Google Scholar] [CrossRef]
- Arslan, S.O.; Gelir, T.E.; Armutcu, F.; Coskun, O.; Gurel, A.; Sayan, H.; Celik, I.L. The protective effect of thymoquinone on ethanol-induced acute gastric damage in the rat. Nutr. Res. 2005, 25, 673–680. [Google Scholar] [CrossRef]
- Kanter, M.; Demir, H.; Karakaya, C.; Ozbek, H. Gastroprotective activity of Nigella sativa L oil and its constituent, thymoquinone against acute alcohol-induced gastric mucosal injury in rats. World J. Gastroenterol. 2005, 11, 6662–6666. [Google Scholar]
- Ohta, Y.; Kobayashi, T.; Ishiguro, I. Participation of xanthine– xanthine oxidase system and neutrophils in development of acute gastric mucosal lesions in rats with a single treatment of compound 48/80, a mast cell degranulator. Dig. Dis. Sci. 1999, 44, 1865–1874. [Google Scholar] [CrossRef]
- Ohta, Y.; Kobayashi, T.; Imai, Y.; Inui, K.; Yoshino, J.; Nakazawa, S. Effect of oral Vitamin E administration on acute gastric mucosal lesion progression in rats treated with compound 48/80, a mast cell degranulator. Biol. Pharm. Bull. 2006, 29, 675–683. [Google Scholar] [CrossRef]
- Singh, S.; Khajuria, A.; Taneja, S.C.; Khajuria, R.K.; Singh, J.; Johri, R.K.; Qazi, G.N. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata, in rats. Phytomedicine 2008, 15, 408–415. [Google Scholar] [CrossRef]
- Kanter, M.; Coskun, O.; Uysal, H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch. Toxicol. 2006, 80, 217–224. [Google Scholar] [CrossRef]
- Chakravarty, N. Inhibition of histamine release from mast cells by nigellone. Ann. Allergy 1993, 70, 237–242. [Google Scholar]
- Magdy, M.A.; Hanan, E.A.; Nabila, E.M. Thymoquinone: Novel gastroprotective mechanisms. Eur. J. Pharmacol. 2012, 697, 126–131. [Google Scholar] [CrossRef]
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: A possible mechanism of action. Cell. Biochem. Funct. 2002, 20, 143–151. [Google Scholar] [CrossRef]
- Al-Ali, A.; Alkhawajah, A.A.; Randhawa, M.A.; Shaikh, N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J. Ayub Med. Coll. Abbottabad 2008, 20, 25–27. [Google Scholar]
- Kacem, R.; Meraihi, Z. Effects of essential oil extracted from Nigella sativa (L.) seeds and its main components on human neutrophil elastase activity. Yakugaku Zasshi 2006, 126, 301–305. [Google Scholar] [CrossRef]
- De Vicenzi, M.; Stammati, A.; de Vicenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. [Google Scholar] [CrossRef]
- Fenaroli, G. Fenaroli’s Handbook of Flavor Ingredients, 4th ed.; CRC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Landa, P.; Kokoska, L.; Pribylova, M.; Vanek, T.; Marsik, P. In vitro anti-inflammatory activity of carvacrol: Inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch. Pharmacal. Res. 2009, 32, 75–78. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.; Rocha, R.F.; Moreira, J.C.; et al. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimaraes, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; Machado, F.D.F.; Quintans-Junior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of Oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef]
- Okabe, S.; Amagase, S. An overview of acetic acid ulcer models: The history and state of the art of peptic ulcer research. Biol. Pharm. Bull. 2005, 28, 1321–1341. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ohta, Y.; Yoshino, J.; Nakazawa, S. Teprenone promotes the healing of acetic acid-induced chronic gastric ulcers in rats by inhibiting neutrophil infiltration and lipid peroxidation in ulcerated gastric tissues. Pharmacol. Res. 2001, 43, 23–30. [Google Scholar] [CrossRef]
- Shahin, M.; Konturek, P.W.; Pohle, T.; Schuppan, D.; Herbst, H.; Domschke, W. Remodeling of extracellular matrix in gastric ulceration. Microsc. Res. Tech. 2001, 5, 396–408. [Google Scholar]
- Oliveira, I.S.; Silva, F.V.; Viana, A.F.S.C.; Santos, M.R.V.; Quintans-Júnior, L.J.; Martins, M.C.C.; Nunes, P.H.M.; Oliveira, F.A.; Oliveira, R.C.M. Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 899–908. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef]
- Raina, V.K.; Kumar, A.; Srivastava, S.K.; Syamsundar, K.V.; Kahol, A.P. Essential oil composition of “kewda” (Pandanus odoratissimus) from India. Flavour. Frag. J. 2004, 19, 434–436. [Google Scholar] [CrossRef]
- Harrathi, J.; Hosni, K.; Karray-Bouraoui, N.; Attia, H.; Marzouk, B.; Magné, C.; Lachaâl, M. Effect of salt stress on growth, fatty acids and essential oils in safflower (Carthamus tinctorius L.). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 34, 129–137. [Google Scholar]
- Choi, Y.J.; Sim, C.; Choi, H.K.; Lee, S.H.; Lee, B.H. α-Terpineol induces fatty liver in mice mediated by the AMP-activated kinase and sterol response element binding protein pathway. Food Chem. Toxicol. 2013, 55, 129–136. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Quintans, J.L.; Almeida, R.N. Evaluation of the Anticonvulsant Activity of alfa-Terpineol. Pharm. Biol. 2007, 45, 69–70. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Oliveira, M.G.B.; Santana, M.F.; Santgana, M.T.; Guimarães, A.G.; Siqueira, J.S.; de Sousa, D.P.; Almeida, R.N. α-terpineol reduces nociceptive behavior in mice. Pharm. Biol. 2011, 49, 583–586. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Porto, D.L.; Menezes, C.P.; Antunes, A.A.; Silva, D.F.; de Sousa, D.P.; Nakao, L.S.; Braga, V.A.; Medeiros, I.A. Unravelling the cardiovascular effects induced by alpha-terpineol: A role for the nitric oxide-cGMP pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 811–816. [Google Scholar]
- Souza, R.H.L.; Cardoso, M.S.P.; Menezes, C.T.; Silva, J.P.; de Sousa, D.P.; Batista, J.S. Gastroprotective activity of α-terpineol in two experimental models of gastric ulcer in rats. Daru 2011, 19, 277–281. [Google Scholar]
- Paisooksantivatana, S.; Bua-in, Y. Essential oil and antioxidant activity of Cassumunar Ginger (Zingiberaceae: Zingiber montanum (Koenig) Link ex Dietr.) collected from various parts of Thailand. Kasetsart J. Nat. Sci. 2009, 43, 467–475. [Google Scholar]
- Sukatta, U.; Rugthaworn, P.; Punjee, P.; Chidchenchey, S.; Keeratinijakal, V. Chemical composition and physical properties of oil from Plai (Zingibercassumunar Roxb.) obtained by hydro distillation and hexane extraction. Kasetsart J. Nat. Sci. 2009, 43, 212–217. [Google Scholar]
- Pazyar, N.; Yaghoobi, R.; Bagheran, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Hamrouni, S.I.; Maamouri, E.; Chahed, T.; Aidi, W.W.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crop. Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Australian Government. Australian Pesticides and Veterinary Medicines Authority. Available online: http://www.apvma.gov.au/advice_summaries/45928.rtf (accessed on 29 April 2014).
- Ninomiya, K.; Hayama, K.; Ishijima, S.A.; Maruyama, N.; Irie, H.; Kurihara, J.; Abe, S. Suppression of inflammatory reactions by terpinen-4-ol, a main constituent of tea tree oil, in a murine model of oral candidiasis and its suppressive activity to cytokine production of macrophages in vitro. Biol. Pharm. Bull. 2013, 36, 838–844. [Google Scholar] [CrossRef]
- Lahlou, S.; Galindo, C.A.B.; Leal-Cardoso, J.H.; Fonteles, M.C.; Duarte, G.P. Cardiovascular effects of the essential oil of Alpinia zerumbet leaves and its main constituent, terpinen-4-ol, in rats: Role of the autonomic nervous system. Planta Med. 2002, 68, 1097–1102. [Google Scholar] [CrossRef]
- Matsunaga, T.; Hasegawa, C.; Kawasuji, T.; Suzuki, H.; Saito, H.; Sagioka, T.; Takahashi, R.; Tsukamoto, H.; Morikawa, T.; Akiyama, T. Isolation of the antiulcer compound in essential oil from the leaves of Cryptomeria japonica. Biol. Pharm. Bull. 2000, 23, 595–598. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Abraham, G.T. Analysis of the essential oil of the roots of the medicinal plant Kaempferia galanga L. (Zingiberaceae) from South India. Acta Pharm. Turc. 2001, 43, 107–110. [Google Scholar]
- Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Almeida, R.N.; Leite, J.R.; Mattei, R. Pharmacological effects of the monoterpene α,β-epoxy-carvone in mice. Braz. J. Pharmacogn. 2007, 17, 170–175. [Google Scholar]
- Almeida, R.N.; Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Araújo, D.A.M.; Leite, J.R.; Mattei, R. Anticonvulsant effect of a natural compound α,β-epoxy-carvone and its action on the nerve excitability. Neurosci. Lett. 2008, 443, 51–55. [Google Scholar] [CrossRef]
- Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R.; Lima, E.O.; Sousa, D.P.; Nunes, X.P.; Pereira, M.S.V.; Barbosa-Filho, J.M.; da Cunha, E.V.L. Preliminary study of the antimicrobial activity of Mentha. x villosa Hudson essential oil, rotundifolone and its analogues. Rev. Bras. Farmacogn. 2006, 16, 307–311. [Google Scholar] [CrossRef]
- Rocha, M.L.; Oliveira, L.E.G.; Santos, C.M.P.; Sousa, D.P.; Almeida, R.N.; Araujo, D.A.M. Antinociceptive and anti-inflammatory effects of the monoterpene α-β-epoxy-carvone in mice. J. Nat. Med. 2013, 67, 743–749. [Google Scholar] [CrossRef]
- Siqueira, B.P.J.; Menezes, C.T.; Silva, J.P.; Sousa, D.P.; Batista, J.S. Antiulcer effect of epoxy-carvone. Braz. J. Pharmacogn 2012, 22, 144–149. [Google Scholar]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Tagami, H. A toxicologic and dermatologic assessment of cyclic and non-cyclic terpene alcohols when used as fragrance ingredientes (Review). Food Chem. Toxicol. 2008, 46, 1–71. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, S.C.; Api, A.M. Fragrance material review on elemol. Food Chem. Toxicol. 2008, 46, 147–148. [Google Scholar] [CrossRef]
- Paluch, G.; Grodnitzky, J.; Bartholomay, L.; Coats, J. Quantitative structure–activity relationship of botanical sesquiterpenes: Spatial and contact repellency to the yellow fever mosquito, Aedes aegypti. J. Agric. Food Chem. 2009, 57, 7618–7625. [Google Scholar] [CrossRef]
- Wedge, D.E.; Tabanca, N.; Sampson, B.J.; Werle, C.; Demirci, B.; Baser, K.H.; Nan, P.; Duan, J.; Liu, Z. Antifungal and insecticidal activity of two Juniperus essential oils. Nat. Prod. Commun. 2009, 4, 123–127. [Google Scholar]
- Vila, R.; Mundina, M.; Muschietti, L.; Priestap, H.A.; Bandoni, A.L.; Adzet, T.; Canigueral, S. Volatile constituents of leaves, roots and stems from Aristolochia elegans. Phytochemistry 1997, 46, 1127–1129. [Google Scholar] [CrossRef]
- Péres, V.F.; Moura, D.J.; Sperotto, A.R.; Damasceno, F.C.; Caramão, E.B.; Zini, C.A.; Saffi, J. Chemical composition and cytotoxic, mutagenic and genotoxic activities of the essential oil from Piper gaudichaudianum Kunth leaves. Food Chem. Toxicol. 2009, 47, 2389–2395. [Google Scholar] [CrossRef]
- Klopell, F.C.; Lemos, M.; Sousa, J.P.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; de Andrade, S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z. Naturforsch. C 2007, 62, 537–542. [Google Scholar]
- Massignani, J.J.; Lemos, M.; Maistro, E.L.; Schaphauser, H.P.; Jorge, R.F.; Sousa, J.P.B.; Bastos, J.K.; Andrade, S.F. Antiulcerogenic Activity of the Essential Oil of Baccharis dracunculifolia on Different Experimental Models in Rats. Phytother. Res. 2009, 23, 1355–1360. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.O.; Lee, J.; Park, D. Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of Bisabolol. Int. J. Toxicol. 1999, 18, 33–44. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, M.; Chittiboyina, A.G.; Avula, B.; Zhao, J.; Khan, I.A. Hydroxylated bisabolol oxides: Evidence for secondary oxidative metabolism in Matricaria chamomilla. J. Nat. Prod. 2013, 76, 1848–1853. [Google Scholar] [CrossRef]
- Vila, R.; Santana, A.I.; Perez-Roses, R.; Valderrama, A.; Castelli, M.V.; Mendonca, S.; Zacchino, S.; Gupta, M.P.; Canigueral, S. Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of alpha-bisabolol. Bioresour. Technol. 2010, 101, 2510–2514. [Google Scholar] [CrossRef]
- Leite, G.O.; Leite, L.H.I.; Sampaio, R.S.; Araruna, M.K.A.; Menezes, I.R.A.; Costa, J.G.M.; Campos, A.R. (−)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia 2011, 82, 208–211. [Google Scholar] [CrossRef]
- Silva, A.P.; Martini, M.V.; Oliveira, C.M.; Cunha, S.; Carvalho, J.E.; Ruiz, A.L.; Silva, C.C. Antitumor activity of (−)-alpha-bisabolol-based thiosemicarbazones against human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar] [CrossRef]
- Bezerra, S.B.; Leal, L.K.A.M.; Nogueira, N.A.P.; Campos, A.R. Bisabolol-induced gastroprotection against acute gastric lesions: Role of prostaglandins, nitric oxide, and KATP+ Channels. J. Med. Food 2009, 12, 1403–1406. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; Venancio, E.T.; Moura, B.A.; Silva, M.I.G.; Aquino Neto, M.F.; Rios, V.E.R.; Sousa, D.P.; Vasconcelos, S.M.M.; Fonteles, M.M.F.; Sousa, F.C.F. Gastroprotection of (−)-α-bisabolol on acute gastric mucosal lesions in mice: The possible involved pharmacological mechanisms. Fundam. Clin. Pharmacol. 2010, 24, 63–71. [Google Scholar] [CrossRef]
- Halici, M.; Odabasoglu, F.; Suleyman, H.; Cakir, A.; Aslan, A.; Bayir, Y. Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine 2005, 12, 656–662. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; Rios, E.R.V.; Carvalho, A.M.R.; Cerqueira, G.S.; Lopes, A.A.; Leal, L.K.A.M.; Dias, M.L.; Sousa, D.P.; Sousa, C.F.C. Anti-nociceptive and anti-inflammatory activities of (−)-α-bisabolol in rodents. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 384, 525–533. [Google Scholar] [CrossRef]
- Dursun, H.; Bilici, M.; Albayrak, F.; Ozturk, C.; Saglam, M.B.; Alp, H.H.; Suleyman, H. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009, 9, 36. [Google Scholar] [CrossRef]
- Sannomiya, M.; Fonseca, V.B.; da Silva, M.A.; Rocha, L.R.; Dos Santos, L.C.; Hiruma-Lima, C.A.; Souza Brito, A.R.; Vilegas, W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J. Ethnopharmacol. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Mates, J.M.; Perez-Gomez, C.; Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Minamiyama, Y.; Ichikawa, H.; Takahashi, S.; Naito, Y.; Kondo, M. Role of lipid peroxidation and antioxidants in gastric mucosal injury induced by the hypoxanthine-x anthine oxidase system in rats. Free Radic. Bio. Med. 1997, 23, 243–250. [Google Scholar] [CrossRef]
- Huang, X.R.; Chun Hui, C.W.; Chen, Y.X. Macrophage migration inhibitory factor is an important mediator in the pathogenesis of gastric inflammation in rats. Gastroenterology 2001, 121, 619–630. [Google Scholar] [CrossRef]
- Chainy, G.B.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: Effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene 2000, 19, 2943–2950. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Mazzanti, G. Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta Med. 2001, 67, 564–566. [Google Scholar] [CrossRef]
- Freire, R.S.; Morais, S.M.; Catunda-Junior, F.E.A.; Pinheiro, D.C.S.N. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorg. Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef]
- Miller, H.E. A simplified method for the evaluation of antioxidant. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Craveiro, A.A.; Andrade, C.H.; Matos, F.J.; Alencar, J.W.; Dantas, T.N. Fixed and volatile constituents of Croton aff. nepetifolius. J. Nat. Prod. 1980, 43, 756–757. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Criddle, D.N.; Leal-Cardoso, J.H. Selective and modulatory effects of the essential oil of Croton zehntneri on isolated smooth muscle preparations of the guinea pig. Phytother. Res. 1998, 12, 189–194. [Google Scholar] [CrossRef]
- Coelho-De-Souza, A.N.; Lahlou, S.; Barreto, J.E.F.; Yum, M.E.M.; Oliveira, A.C.; Oliveira, H.D.; Celedonio, N.R.; Feitosa, R.G.R.; Duarte, G.P.; Santos, C.F.; et al. Essential oil of Croton zehntneri and its major constituent anethole display gastroprotective effect by increasing the surface mucous layer. Fundam. Clin. Pharmacol. 2013, 27, 288–298. [Google Scholar] [CrossRef]
- Hagan, E.C.; Jenner, P.M.; Jones, W.I.; Fitzhugh, O.G.; Long, E.L.; Brouwer, J.G.; Dwebb, W.K. Toxic properties of compounds related to safrole. Toxic. Appl. Pharmac. 1965, 7, 18–24. [Google Scholar] [CrossRef]
- Taylor, J.M.; Jenner, P.M.; Jones, W.I. A comparison of the toxicity of some allyl, propenyl and propyl compounds in the rat. Toxic. Appl. Pharm. 1964, 2, 378. [Google Scholar] [CrossRef]
- Truhaut, R.; Le Bourhis, B.; Attia, M.; Glomot, R.; Newman, J.; Caldwell, J. Chronic toxicity/carcinogenicity study of trans-anethole in rats. Food Chem. Toxicol. 1989, 27, 11–20. [Google Scholar] [CrossRef]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- Sober, H.A.; Hollander, F.; Sober, E.K. Toxicity of eugenol determination of LD50 on rats. Exp. Biol. Med. 1950, 73, 148–151. [Google Scholar] [CrossRef]
- Sax, N.I. Dangerous Properties of Industrial Materials, 11th ed.; Lewis, R.J., Sr., Ed.; Wiley-Interscience; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; p. 1735. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, E.H.; Kim, C.Y.; Chung, J.G.; Kim, S.H.; Lim, J.P.; Shin, T.Y. Antianaphylactic properties of eugenol. Pharmacol. Res. 1997, 36, 475–680. [Google Scholar] [CrossRef]
- Sharma, J.N.; Srivastava, K.C.; Gan, E.K. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology 1994, 49, 314–318. [Google Scholar] [CrossRef]
- Santin, J.R.; Lemos, M.; Klein-Júnior, L.C.; Machado, I.D.; Costa, P.; Oliveira, A.P.; Tilia, C.; Souza, J.P.; Sousa, J.P.B.; Bastos, J.K.; et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 149–158. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Santos, L.C.; Kushima, H.; Pellizzon, C.H.; Silveira, G.G.; Vasconcelos, P.C.P.; Vilegas, W.; Souza Brito, A.R.M. Qualea grandiflora, a Brazilian “Cerrado” medicinal plant presents an important antiulcer activity. J. Ethnopharmacol. 2006, 104, 207–214. [Google Scholar] [CrossRef]
- Capasso, R.; Pinto, L.; Vuotto, M.L.; di Carlo, G. Preventive effect of eugenol on PAF and ethanol-induced gastric mucosal damage. Fitoterapia 2000, 71, 131–137. [Google Scholar] [CrossRef]
- Maity, P.; Biswas, K.; Roy, S.; Banerjee, R.K.; Bandyopadhyay, U. Smoking and the pathogenesis of gastroduodenal ulcer—recent mechanistic update. Mol. Cel. Biochem. 2003, 253, 329–338. [Google Scholar] [CrossRef]
- Braquet, P.; Etienne, A.; Mencia-Huerta, J.M.; Clostre, F. Effects of the specific platelet-activating factor antagonists, BN 52021 and BN 52063, on various experimental gastrointestinal ulcerations. Eur. J. Pharmacol. 1988, 150, 269–276. [Google Scholar] [CrossRef]
- Morsy, M.A.; Fouad, A.A. Mechanisms of gastroprotective effect of eugenol in indomethacin-induced ulcer in rats. Phytother. Res. 2008, 22, 1361–1366. [Google Scholar] [CrossRef]
- Souza, M.H.L.P.; Lemos, H.P.; Oliveira, R.B.; Cunha, F.Q. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut 2004, 53, 791–796. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Kishi, A.; Tomii, T.; Kaneko, T.; Iinuma, S.; Ichikawa, H.; Yasuda, M.; Takahashi, S.; Kondo, M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993, 34, 732–737. [Google Scholar] [CrossRef]
- Goel, R.K.; Bhattacharya, S.K. Gastroduodenal mucosal defense and protective agents Indian. J. Exp. Biol. 1991, 29, 701–714. [Google Scholar]
- Iwai, T.; Ichikawa, T.; Goso, Y.; Ikezawa, T.; Saegusa, Y.; Okayasu, Y.; Saigenji, K.; Ishihara, K. Effects of indomethacin on the rat small intestinal mucosa: Immunohistochemical and biochemical studies using anti-mucin monoclonal antibodies. J. Gastroenterol. 2009, 44, 277–284. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.H.; Bae, K.H.; Jeong, C.S. Anti-gastric actions of eugenol and cinnamic acid isolated from Cinnamoni ramulus. Yakugaku Zasshi 2011, 131, 1103–1110. [Google Scholar] [CrossRef]
- Marhuenda, E.; Martin, M.J.; Alarcon De La Lastra, C. Antiulcerogenic activity of aescine in different experimental models. Phytother. Res. 1993, 7, 13–16. [Google Scholar] [CrossRef]
- Yamahara, J.; Mochizuki, M.; Rong, H.Q.; Matsuda, H.; Fujimura, H. The anti-ulcer effect in rats of ginger constituents. J. Ethnopharmacol. 1988, 23, 299–304. [Google Scholar] [CrossRef]
- Yang, X.; Eilerman, R.G. Pungent Principal of Alpinia. galangal (L.) Swartz and Its Applications. J. Agric. Food Chem. 1999, 47, 1657–1662. [Google Scholar] [CrossRef]
- Nakamura, Y.; Murakami, A.; Ohto, Y.; Torikai, K.; Tanaka, T.; Ohigashi, H. Suppression of tumor promoter-induced oxidative stress and inflammatory responses in mouse skin by a superoxide generation inhibitor 1V-acetoxychavicol acetate. Cancer Res. 1998, 58, 4832–4839. [Google Scholar]
- Kubota, K.; Ueda, Y.; Yasuda, M.; Masuda, A. Occurrence and antioxidative activity of 1V-acetoxychavicol acetate and its related compounds in the rhizomes of Alpinia galanga during cooking. Spec. Publ. R. Soc. Chem. 2001, 274, 601–607. [Google Scholar]
- Mitsui, S.; Kobayashi, S.; Nagahori, H.; Ogiso, A. Constituents from seeds of Alpinia galanga WILD and their anti-ulcer activies. Chem. Pharm. Bull. 1976, 24, 2377–2382. [Google Scholar] [CrossRef]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Ochi, M.; Yoshikawa, M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: Structural requirements and mode of action. Eur. J. Pharmacol. 2003, 471, 59–67. [Google Scholar] [CrossRef]
- Gruenwald, J.; Freder, J.; Armbruester, N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2012, 50, 822–834. [Google Scholar]
- Leach, M.J.; Kumar, S. Cinnamon for diabetes mellitus. Cochrane Database Syst. Rev. 2012, 9. [Google Scholar] [CrossRef]
- Keshvari, M.; Asgary, S.; Jafarian-Dehkordi, A.; Najafi, S.; Ghoreyshi-Yazdi, S.M. Preventive effect of cinnamon essential oil on lipid oxidation of vegetable oil. Atherosclerosis 2013, 9, 280–286. [Google Scholar]
- Mereto, E.; Brambilla-Campart, G.; Ghia, M.; Martelli, A.; Brambilla, G. Cinnamaldehyde-induced micronuclei in rodent liver. Mutat. Res. 1994, 322, 1–8. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Basir, S.F.; Manzoor, N.; Khan, L.A. Spice oil cinnamaldehyde exhibits potent anticandidal activity against fluconazole resistant clinical isolates. Fitoterapia 2010, 82, 1012–1020. [Google Scholar]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid. Based Complement. Altern. Med. 2012, 429320. 1–12. [Google Scholar]
- Kim, B.H.; Lee, Y.G.; Lee, J.; Lee, J.Y.; Cho, J.Y. Regulatory effect of cinnamaldehyde on monocyte/macrophage-mediated inflammatory responses. Mediat. Inflamm. 2010, 2010, 1–9. [Google Scholar]
- Wondrak, G.T.; Villeneuve, N.F.; Lamore, S.D.; Bause, A.S.; Jiang, T.; Zhang, D.D. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules 2010, 15, 3338–3355. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Fahmy, A.; Badawy, D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem. Toxicol. 2011, 49, 3007–3012. [Google Scholar] [CrossRef]
- Tankam, J.M.; Sawada, Y.; Ito, M. Regular ingestion of Cinnamomi cortex pulveratus offers gastroprotective activity in mice. J. Nat. Med. 2013, 67, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Harada, M.; Yano, S. Pharmacological studies on Chinese cinnamon. Effects of cinnamaldehyde on the cardiovascular and digestive systems. Chem. Pharm. Bull. 1975, 23, 941–947. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef]
- Levenstein, I. Acute Oral Toxicity Study in Rats and Acute Dermal Toxicity Study in Rabbits; Unpublished Report to Research Institute for Fragrance Materials Inc.: Englewood Cliffs, NJ, USA, 1976. [Google Scholar]
- Zaitsev, A.N.; Rakhmanina, N.L. Toxic properties of pheny-lethanol and cinnamic alcohol derivatives. Vopr. Pitan. 1974, 5, 48–53. [Google Scholar]
- Conti, B.R.; Búfalo, M.C.; Golim, M.A.; Bankova, V.; Sforcin, J.M. Cinnamic acid is partially involved in propolis immunomodulatory action on human monocytes. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–7. [Google Scholar]
- Villena, J.; Kitazawa, H. Modulation of intestinal TLR4-inflammatory signaling pathways by probiotic microorganisms: Lessons learned from Lactobacillus jensenii TL2937. Front. Immunol. 2014, 4, 512. [Google Scholar]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-Anun, S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef]
- Furia, T.E.; Bellanca, N. Fenaroli’s Handbook of Flavor Ingredients; Chemical Rubber Company Press: Cleveland, OH, USA, 1975; p. 2. [Google Scholar]
- Ress, N.B.; Hailey, J.R.; Maronpot, R.R.; Bucher, J.R.; Travlos, G.S.; Haseman, J.K.; Orzech, D.P.; Johnson, J.D.; Hejtmancik, M.R. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 2003, 71, 198–206. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Ramírez-Montiel, M.L.; González-García, M.P.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Cariño-Cortés, R. The combination of naproxen and citral reduces nociception and gastric damage in rats. Arch. Pharm. Res. 2010, 10, 1691–1697. [Google Scholar]
- Derby, R.; Rohal, P.; Jackson, C.; Beutler, A.; Olsen, C. Novel treatment of onychomycosis using over-the-counter mentholated ointment: A clinical case series. J. Am. Board Fam. Med. 2011, 24, 69–74. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Cherrat, L.; Espina, L.; Loran, S.; Rota, C.; Pagan, R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov. Food Sci. Emerg. 2011, 12, 320–329. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Wang, D.C.; Xiang, H.; Feng, H.H.; Jiang, Y.S.A.; Xia, L.J. Subinhibitory concentrations of thymol reduce enterotoxins A and B and alpha-hemolysin production in Staphylococcus aureus isolates. PLoS One 2010, 5, e9736. [Google Scholar]
- Dhaneshwar, S.; Patel, V.; Patil, D.; Meena, G. Studies on synthesis, stability, release and pharmacodynamic profile of a novel diacerein-thymol prodrug. Bioorg. Med. Chem. Lett. 2013, 23, 55–61. [Google Scholar] [CrossRef]
- Rintelen, B.; Neumann, K.; Leeb, B.F. A meta-analysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch. Intern. Med. 2006, 166, 1899–1906. [Google Scholar] [CrossRef]
- Rainsford, K.D. Aspirin and gastric ulceration: Light and electron microscopic observations in a model of aspirin plus stress-induced ulcerogenesis. Br. J. Exp. Pathol. 1977, 58, 215–219. [Google Scholar]
- Jung, H.W.; Mahesh, R.; Park, J.H.; Boo, Y.C.; Park, K.M.; Park, Y.K. Bisabolangelone isolated from Ostericum koreanum inhibits the production of inflammatory mediators by down-regulation of NF-kappaB and ERK MAP kinase activity in LPS-stimulated RAW264.7 cells. Int. Immunopharmacol. 2010, 10, 155–162. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Zou, K.; Cheng, F.; Dan, F.; Guo, Z.; Cai, Z.; Yang, J. The antiulcer activities of bisabolangelone from Angelica polymorpha. J. Ethnopharmacol. 2009, 123, 343–346. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, Y.J.; Lee, H.K.; Kim, J.S.; Park, Y.; Kang, J.S.; Hwang, B.Y.; Hong, J.T.; Kim, Y.; Han, S.B. Bisabolangelone inhibits dendritic cell functions by blocking MAPK and NF-jB signaling. Food Chem. Toxicol. 2013, 59, 26–33. [Google Scholar] [CrossRef]
- Zhan, Y. The Medicinal Source of China Shennongjia; The Science and Technological Press of Hubei: Hubei, China, 1994; pp. 418–419. [Google Scholar]
- Fang, Z.; Liao, Z. The Medicinal Plants from Enshi of Hubei; The Science and Technological Press of Hubei: Hubei, China, 2006; p. 116. [Google Scholar]
- Hata, K.; Kozawa, M.; Ikeshiro, Y. On the coumarins of the roots of Angelica polymorpha Maxim. (Umbelliferae). Yakugaku Zasshi 1967, 87, 464–465. [Google Scholar]
- Yang, Y.; Zhang, Y.; Ren, F.X.; Yu, N.J.; Xu, R.; Zhao, Y.M. Chemical constituents from the roots of Angelica polymorpha Maxim. Yao Xue Xue Bao. 2013, 48, 718–722. [Google Scholar]
- Cai, Z.J.; Dan, F.J.; Cheng, F.; Wang, J.Z.; Zou, K. Chemical constituents of antibacterial activity fraction of Angelica polymorpha. Zhong Yao Cai 2008, 31, 1160–1162. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oliveira, F.D.A.; Andrade, L.N.; De Sousa, É.B.V.; De Sousa, D.P. Anti-Ulcer Activity of Essential Oil Constituents. Molecules 2014, 19, 5717-5747. https://doi.org/10.3390/molecules19055717
Oliveira FDA, Andrade LN, De Sousa ÉBV, De Sousa DP. Anti-Ulcer Activity of Essential Oil Constituents. Molecules. 2014; 19(5):5717-5747. https://doi.org/10.3390/molecules19055717
Chicago/Turabian StyleOliveira, Francisco De Assis, Luciana Nalone Andrade, Élida Batista Vieira De Sousa, and Damião Pergentino De Sousa. 2014. "Anti-Ulcer Activity of Essential Oil Constituents" Molecules 19, no. 5: 5717-5747. https://doi.org/10.3390/molecules19055717