2. Results and Discussion
In the context of the growing general interest for reducing energy costs, heating chemical reactions under microwave irradiation is a useful approach for achieving higher reaction kinetics and synthesizing cleaner products [
10,
11,
12,
13]. In combination with microwave technology and lipases, we wished to examine the synthesis of chiral cyclooctenic diols and diesters starting from cycloocta-1,5-diene using microwave technology.
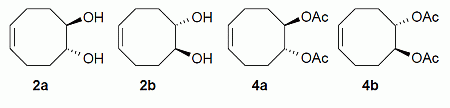
rac-Diols
2 and rac-diacetates
4 were initially prepared from cycloocta-1,5-diene in a three-steps sequence including epoxidation, ring opening with aqueous sulfuric acid, followed by acetylation with acetic anhydride (
Scheme 1) [
14,
15].
Scheme 1.
Epoxidation and hydrolysis of cycloocta-1,5-diene leading to racemic diols 2 and acetylation affording diacetates 4.
Scheme 1.
Epoxidation and hydrolysis of cycloocta-1,5-diene leading to racemic diols 2 and acetylation affording diacetates 4.
The preparation of optically active 5-cyclooctene-1,2-diol
2 was first conceived by using microwave-assisted lipase-catalyzed desymmetrization of
meso-symmetric diol
2 using vinyl acetate as the acylating agent in THF as solvent rather than isooctane or 2-methylbutan-2-ol, that gave lower results. In order to perform the reaction under microwave irradiation, we decided to choose thermostable immobilized lipases capable of withstanding microwave irradiation: Novozyme 435
® (CaLB immobilized on acrylic resin) and PS-D (
Pseudomonas cepacia immobilized on diatomite). The goal was to obtain an enantioselective enhancement with immobilized lipases, as previous studies performed in our laboratory showed that resolution of
rac-diol
2 with free
Pseudomonas cepacia lipase at 55 °C in THF during 7 days afforded with a good conversion
rac-monoacetate
3 (47%, 0% ee) and
rac-diol
2 (51%, 0% ee) but with no selectivity at all. Indeed, by immobilization of enzymes onto solid supports, enhanced enzyme activity, selectivity, stability, and reusability in organic media may be achieved compared to the native enzyme [
16].
Performed at 35 °C under conventional heating immobilized CaLB-enzymatic acylation of
rac-diol (
2) afforded after 3 weeks 28% of (1
R,2
R)-monoacetate
3a (42% ee) and 6% of (1
S,2
S)-diacetate
4b with an excellent 99% ee. A higher temperature (50 °C) led after 7 days to modest yields of monoacetate
3a and a real enhancement of yield for diacetate
4b (20% with ee > 99%) (
Scheme 2 and
Table 1).
Scheme 2.
Enantioselective acetylation of diol (2) using immobilized CaLB lipase and vinyl acetate by classical heating at various temperatures.
Scheme 2.
Enantioselective acetylation of diol (2) using immobilized CaLB lipase and vinyl acetate by classical heating at various temperatures.
Table 1.
Enantioselective acetylation of diol (2) using immobilized CaLB lipase and vinyl acetate by classical heating at various temperatures.
Table 1.
Enantioselective acetylation of diol (2) using immobilized CaLB lipase and vinyl acetate by classical heating at various temperatures.
| Heating Mode | Temperature (°C) | Time | Monoacetate 3a Yield (%) | ee (%) | Diacetate 4b Yield (%) | ee (%) |
|---|
| Classical | 35 | 3 weeks | 28 | 42 | 6 | >99 |
| Classical | 50 | 7 days | 30 | 50 | 20 | >99 |
| Classical | 50 | 14 h | traces | - |
Under microwave irradiation at 35 °C (5 W), racemic diol
2 proceeded to give (1
R,2
R)-monoacetate
3a (32%, 45% ee), trace amounts of (1
S,2
S)-diacetate
4b (5%, >99% ee) and 65% of diol
2 (23% ee). In order to study the influence of the irradiation power on the biocatalytic media, we decided to apply a constant power (up to 300 W) while maintaining the temperature at 35 °C by using a microwave oven combined with a Coolmate
®. We noticed an enhancement of the yield and the enantiomeric ratio of (1
R,2
R)-monoacetate
3a (42%, 67% ee) and diol
2 (51%, 50% ee) and only 2% of diacetate
4b (99% ee). At 50 °C (10W, 14 h) the same reaction yielded only esters: 58% of (1
R,2
R)-monoacetate
3a with 55% ee and 37% of diacetate
4b (99% ee). A higher temperature (80 °C, 40 W, 14 h) afforded a decrease of diacetate yield with a lower enantiomeric excess (30% of
4b with 94% ee) (
Scheme 3 and
Table 2).
Scheme 3.
Enantioselective acetylation of diol 2 using immobilized CaLB with vinyl acetate lipase under microwave irradiation.
Scheme 3.
Enantioselective acetylation of diol 2 using immobilized CaLB with vinyl acetate lipase under microwave irradiation.
Table 2.
Enantioselective acetylation of diol 2 using immobilized CaLB lipase under microwave irradiation in a large range of temperatures. * Constant power when the temperature is reached (max: 300 W, 2 min); ** Using Coolmate.
Table 2.
Enantioselective acetylation of diol 2 using immobilized CaLB lipase under microwave irradiation in a large range of temperatures. * Constant power when the temperature is reached (max: 300 W, 2 min); ** Using Coolmate.
| Power (W) | Incubation Time (h) | Temperature (°C) | Monoacetate 3a | Diaceate 4b |
|---|
| yield (%) | ee (%) | yield (%) | ee (%) |
|---|
| 5 * | 14 | 35 | 32 | 45 | 5 | 99 |
| 10 * | 14 | 50 | 58 | 55 | 37 | 99 |
| 20 * | 14 | 80 | 55 | 57 | 30 | 94 |
| 40 * | 14 | 100 | - | - | - | - |
| 300 ** | 14 | 35 | 42 | 67 | 2 | >99 |
These results suggest that, at higher temperature (100 °C), there is a loss of enzyme activity due to its denaturation. At 80 °C, Poojari
et al., have shown that the immobilized-CalB (Novozym 435) was very stable and kept 90% of its activity after an incubation in diphenylether for 24 h [
17]. The irradiation power appears to display a key role in enzyme properties, and best enhancement of monoacetate yield and ee was observed at 35 °C with 300 W. Brimble
et al., have shown that compared to conventional heating the microwave irradiation led to higher conversion and enantiomeric excesses, in the case of lipase-catalyzed kinetic resolution of racemic secondary alcohols through acetylation [
18].
At 50 °C under conventional heating, enzyme-catalyzed acylation of rac-diol
2 with immobilized PS-D provided both (1
S,2
S)-monoacetate
3b and (1
R,2
R)-diol
2a in poor enantiomeric purity (respectively 45% ee for
3b and 3% ee for
2a) and poor conversion (6%). At higher temperatures (80, 100 °C), PS-D proved to be ineffective and lost all enzymatic activity. Under microwave irradiation at 50 °C (15 W), lipase resolution of racemic diol
2 afforded (1
S,2
S)-monoacetate
3b (41%, 50% ee) and (1
R,2
R)-diol (57%, 35% ee). The microwave irradiation method gave a higher conversion compared to conventional heating. At 80 °C (35 W), the reaction yielded 12% of
3b (35% ee) and 66% of diol with no selectivity (5% ee) (
Scheme 4 and
Table 3).
Scheme 4.
Acetylation of diol 2 using immobilized PS lipase (PS-D).
Scheme 4.
Acetylation of diol 2 using immobilized PS lipase (PS-D).
Table 3.
Acetylation of diol 2 using immobilized PS lipase (PS-D) (incubation time:14 h). Effect of the heating mode and temperature on the conversion and kinetic resolution of rac-2 into diol 2a and monoacetate 3b. * Constant power when the temperature is reached (max:300 W and 2 min).
Table 3.
Acetylation of diol 2 using immobilized PS lipase (PS-D) (incubation time:14 h). Effect of the heating mode and temperature on the conversion and kinetic resolution of rac-2 into diol 2a and monoacetate 3b. * Constant power when the temperature is reached (max:300 W and 2 min).
| Heating Mode | Temperature (°C) | Diol 2aee (%) | Monoacetate 3b yield (%) | ee (%) |
|---|
| Classical | 50 | 3 | 6 | 45 |
| 80 | - | - | - |
| 100 | - | - | - |
| Microwave | 50 (15 W *) | 35 | 41 | 50 |
| 80 (35 W *) | 5 | 12 | 35 |
| 100 (closed vessel) (40 W *) | - | - | - |
Following these preliminary results, we then investigated the lipase-catalyzed kinetic hydrolytic resolution of
rac-diacetate
4 according to the Suemune procedure [
19]. Instead of using PFL (
Pseudomonas fluorescens lipase), we used immobilized CaLB and PS-D in order to perform later the reaction under microwave. Performed at various temperature (35, 50 and 80 °C) under conventional heating CaLB-catalyzed hydrolysis of
rac-diacetate (
4) in phosphate buffer (0.1 M, pH 7.0) led only to traces of monoacetate at 50 °C. Compared to conventional heating, the microwave irradiation at 50 °C during 14 h led to a higher conversion (20%) with excellent enantiomeric excess for monoacetate (ee: 97% for
3b (1
S,2
S)) besides diacetate
4a (ee: 34%) (
Scheme 5 and
Table 4).
Scheme 5.
Hydrolysis of diacetate
4 using immobilized CaLB lipase in phosphate buffer (0.1 M, pH 7). For detailed conversion and ee, see
Table 4.
Scheme 5.
Hydrolysis of diacetate
4 using immobilized CaLB lipase in phosphate buffer (0.1 M, pH 7). For detailed conversion and ee, see
Table 4.
Table 4.
Hydrolysis of rac-diacetate 4 using immobilized CaLB lipase (incubation time: 14 h). Effect of the heating mode and temperature on the conversion and kinetic resolution of rac-4 into diacetate 4a and monoacetate 3b. * Constant power when the temperature is reached (max: 300 W and 2 min).
Table 4.
Hydrolysis of rac-diacetate 4 using immobilized CaLB lipase (incubation time: 14 h). Effect of the heating mode and temperature on the conversion and kinetic resolution of rac-4 into diacetate 4a and monoacetate 3b. * Constant power when the temperature is reached (max: 300 W and 2 min).
| Heating Mode | Temp. (°C) | Conversion | Diacetate 4a ee (%) | Monoacetate 3b ee (%) |
|---|
| Classical | 35 | - | - | - |
| 50 | Traces | - | - |
| 80 | - | - | - |
| Microwave | 35 (1–5 W *) | - | - | - |
| 50 (5 W *) | 20% | 34 | 97 |
| [α]D = +29° | [α]D = +6° |
| 80 (10 W *) | - | - | - |
Given the promising results obtained during the first enantioenrichment of
rac-
4 with CaLB, the recovered (1
R,2
R)-diacetate
4a with 34% ee was submitted twice to the same enzymatic hydrolysis under microwave irradiation (2 × 14 h, 50 °C, 5 W). Enantiopure (1
R,2
R)-diacetate
4a was obtained in 49% yield from
rac-
4 (
Scheme 6 and
Table 5).
Scheme 6.
Sequential enantioenrichment of diacetate 4a.
Scheme 6.
Sequential enantioenrichment of diacetate 4a.
Table 5.
Sequential enantioenrichment of diacetate 4a.
Table 5.
Sequential enantioenrichment of diacetate 4a.
| | Yield (%) | Monoacetate 3b ee (%) | [α]D | yield (%) | Diacetate 4a ee (%) | [α]D |
|---|
| 1st enrichment | 20 | 97 | +5.8° | 80 | 34 | +25° |
| 2nd enrichment | 41 | 98 | +6° | 57 | 72 | +57° |
| 3rd enrichment | 51 | 97 | +5.7° | 49 | >99 | +81° |
CaLB and the PS-D were finally compared for their ability to carry out an effective kinetic resolution of rac-
4 diester. The hydrolysis using PS-D was conducted in the same manner. In classical conditions whatever the temperature (35, 50, 80 °C) and the reaction time (14 h, 7 days), in no case conversion to monoacetate
3 was observed. Interestingly, under microwave irradiation at 50 °C (14 h),
rac-
4 was desymmetrized into (1
R,2
R) monoacetate
3a with 81% ee and (1
S,2
S)-diacetate
4b (28% ee), highlighting a non-thermal effect (
Scheme 7 and
Table 6).
The microwave-assisted hydrolysis seems to enhance the enzyme activity compared to classical heating. Comparing the results with Suemune
et al. [
19], the CaLB seems to be a convenient enzyme in microwave irradiation resulting in the obtention of enantiopure monoacetate
3a in a quick and clean way. However, the same reaction with PS-D needed to be optimized to obtain a better ee.
Scheme 7.
Hydrolysis of diacetate 4 using PS-D lipase in phosphate buffer (0.1 M, pH 7).
Scheme 7.
Hydrolysis of diacetate 4 using PS-D lipase in phosphate buffer (0.1 M, pH 7).
Table 6.
Hydrolysis of diacetate 4 using PS-D lipase (incubation time: 14 h). Effect of the heating mode and temperature on the conversion and kinetic resolution of rac-4 into monoaceate 3a and diester 4b. * Constant power when the temperature is reached (max: 300 W and 2 min).
Table 6.
Hydrolysis of diacetate 4 using PS-D lipase (incubation time: 14 h). Effect of the heating mode and temperature on the conversion and kinetic resolution of rac-4 into monoaceate 3a and diester 4b. * Constant power when the temperature is reached (max: 300 W and 2 min).
| Mode of Heating | Temp. (°C) | Conversion | Monoacetate 3a ee (%) | Diacetate 4b ee (%) |
|---|
| Classical | 35 | - | - | - |
| 50 | - | - | - |
| 80 | - | - | - |
| Microwave | 35 (1–5 W *) | - | - | - |
| 50 (5 W *) | 10% | 81 | 28 |
| | | [α]D = −4° | [α]D = −24° |
| 80 (10 W *) | - | - | - |
Finally, the isolated enantiopure monoacetates
3a,
3b and diacetates
4a and
4b were then quantitatively converted into (1
R,2
R)-diol
2a and (1
S,2
S)-diol
2b with >99% ee by methanolysis [
7] (
Scheme 8).
Scheme 8.
Isolation of enantiopure diols 2a and 2b by methanolysis of monoacetate 3a and diacetate 4b.
Scheme 8.
Isolation of enantiopure diols 2a and 2b by methanolysis of monoacetate 3a and diacetate 4b.
3. Experimental Section
3.1. General Information
Lipases from Pseudomonas cepacia (immobilized on diatoms MKBB3465, 500 PLU−1) and Candida antarctica (immobilized on acrylic resin 077K1155, 10,000 PLU−1 Novozym 435® or free form) were purchased from Sigma Aldrich (Sigma-Aldrich Chemie S.a.r.l., Saint Quentin Fallavier, France). All other chemicals were purchased from Sigma Aldrich and were used without further purification except in the case of vinyl acetate which was used after fresh distillation.
IR spectra were recorded on a Perkin–Elmer Spectrum 100 IRFT-ATR instrument (Perkin-Elmer, Courtaboeuf-Les Ulis, France). 1H- and 13C-NMR were recorded on a JEOL JNM LA400 (400 MHz) spectrometer (JEOL SAS Europe, Croissy Sur Seine, France). Chemical shifts (δ) are reported in parts per million (ppm) downfield from tetramethylsilane (TMS) which was used as internal standard. Coupling constants J are given in Hz. The high resolution mass spectra (HRMS) were recorded on a Varian MAT311 spectrometer (Agilent Technologies SAS, Les Ulis, France) in the Centre Régional de Mesures Physiques de l’Ouest (CRMPO), Université de Rennes. Analytical thin layer chromatography (TLC) was performed on Merck Kieselgel 60 F254 aluminum packed plates (Merck KgaA, Darmstadt, Germany).
Enantiomeric ratios were determined by gas chromatography (Agilent 7890A, Agilent Technologies SAS, Les Ulis, France) equipped with an autosampler (7688B) and flame ionization detector (FID). For the experiment, a CP-Chirasil-Dex (0.25 mm × 25 m × 0.25 µm, Chromopack, Agilent Technologies SAS, Les Ulis, France) column was used. The injector and the detector were kept at 180 °C. Nitrogen was used as gas carrier at a flow of 1.5 mL/min. Hydrogen, air and nitrogen were supplied to the FID at 35 mL·min−1, 350 mL·min−1 and 25 mL·min−1 respectively. The products are analyzed at 110 °C. The enantiomeric ratio and yields were calculated by taking the average of two duplicates, with an error <2%.
High performance liquid chromatography (HPLC) was carried out in a Waters 600 s system (Water SAS, Guyancourt, France) combined with an autosampler (Waters 717 plus). A Chiralpak–AD column (amylose tris-(3,5-dimethylphenylcarbamate) coated on 10 μm silica-gel, Daicel Chemical, 250 × 4.6 mm, Chiral Technologies Europe SAS, Illkirch, France) is used. Eluent was n-heptane/EtOH, 9/1 at a flowrate of 1 mL·min−1. Products were analyzed using a differential refractometer (Waters 410). Optical rotations were measured on a Perkin Elmer 341 polarimeter.
Microwave reactions were conducted using a CEM Discover®, single mode operating system (CEM France, Saclay, France) working at 2.45 GHz, with a programmable power ranging from 1 to 300 W. The microwave can be equipped with a Coolmate® system allowing reactions with high power input (up to 300 W) while maintaining the reaction media at 35 °C. This system is cooled by a cryogenic fluid (Galden HT-55®, BT Electronics, Les Ulis, France). In closed vessel mode, microwave irradiation experiments were carried out using a single-mode microwave instrument (Initiator, Biotage, Uppsala, Sweden) working at 2.45 GHz, with a power programmable from 1 to 450 W (0–20 bars).
3.2. (Z)-(1S,8R)-9-Oxa-Bicyclo[6.1.0]Non-4-Ene (1)
Under inert atmosphere, to a solution of cycloocta-1,5-diene (20 g, 0.161 mol) and sodium carbonate (117.6 g, 1.11 mol) in dichloromethane (560 mL) stirred at 0 °C was added dropwise a solution of peracetic acid (42.7 mL, 0.222 mol) in dichloromethane (500 mL) during 2 h. The mixture was stirred for 8 h at 0 °C and 12 h at room temperature. The mixture was quenched with 500 mL of water and extracted with (3 × 250 mL) of dichloromethane. The organic layers were dried with magnesium sulfate anhydrous and concentrated under reduced pressure. The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 90/10) to provide the (Z)-(1S,8R)-9-oxa-bicyclo[6.1.0]non-4-ene (1, 5.697 g, 67% yield) as a colorless oil; νmax (cm−1): 3003, 2906, 2887, 1655, 1445, 1428, 1228, 1669, 1099, 1039, 934, 762, 743; 1H-NMR (CDCl3): δH 5.56–5.60 (2H, m, CH = CH), 3.00–3.02 (2H, m, CHCH), 2.41–2.47 (2H, m, CH2), 2.08–2.15 (2H, m, CH2), 1.98–2.17 (4H, m, 2×CH2); 13C-NMR (CDCl3): δC 128.6, 56.2, 27.8, 23.4.
3.3. (Z)-Cyclooct-5-en-1,2-diol (2)
Under inert atmosphere, to the (Z)-(1S,8R)-9-oxabicyclo[6.1.0]non-4-ene (1, 5.697 g, 45.9 mmol) vigorously stirred was added a solution of sulfuric acid 2 M (25.3 mL, 50.47 mmol). After 4 h under stirring, the mixture was extracted with 3 × 75 mL of ethyl acetate. The organic layers were washed with a saturated solution of sodium hydrogenocarbonate (40 mL), brine (40 mL), dried with magnesium sulfate and concentrated under vacuum. The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 60/40) to give the rac-(Z)-cyclooct-5-en-1,2-diol (2, 3.691 g, 57% yield) as a colorless oil; νmax (cm−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719; 1H-NMR (CDCl3): δH 5.55–5.59 (2H, m, CH = CH), 3.57–3.61 (4H, m, CHOHCHOH), 2.29–2.35 (2H, m, CH2), 2.00–2.12 (4H, m, 2×CH2), 1.52–1.58 (2H, m, CH2); 13C-NMR (CDCl3): δC 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C8H14O2 [M]+: 142.09938, Found: 142.1001 (5 ppm).
3.4. (1R,2R)-(Z)-1-Hydroxycyclooct-4-enyl Acetate (3a)
Compound
3a was obtained as a colorless oil
![Molecules 19 09215 i001]()
−3.0° (
c 1.00 CHCl
3). ν
max (cm
−1): 3449, 3012, 2936, 1717, 1654, 1430, 1235, 1030, 971, 933, 720;
1H-NMR (CDCl
3): δ
H 5.59–5.69 (2H, m, C
H = C
H), 4.95 (1H dt,
J = 8.8 Hz, 4.0Hz, C
HOAc), 3.90 (1H, dt,
J = 2.1 Hz, C
HOH), 2.54 (1H, s, O
H), 2.38–2.42 (m, 2H, C
H2), 2.04–2.25 (m, 7H, 2×C
H2 and C
H3), 1.68–1.75 (m, 2H, C
H2);
13C-NMR (CDCl
3): δ
C 170.9, 129.6, 128.6, 77.3, 72.1, 32.8, 30.0, 22.8, 22.8, 21.2. GC: Cromopack column t
R = 59.7 min HRMS: calcd for C
8H
14O
2 [M-CH
2CO]
+: 142.09938, Found: 142.0993 (0 ppm).
3.5. (1S,2S)-(Z)-1-Hydroxycyclooct-4-enyl Acetate (3b)
Compound
3b was obtained as a colorless oil.
![Molecules 19 09215 i001]()
+3.2° (
c 1.00 CHCl
3). ν
max (cm
−1): 3449, 3012, 2936, 1717, 1654, 1430, 1235, 1030, 971, 933, 720;
1H-NMR (CDCl
3): δ
H 5.59–5.69 (2H, m, C
H = C
H), 4.95 (1H dt,
J = 8.8 Hz, 4.0Hz, C
HOAc), 3.90 (1H, dt,
J = 2.1 Hz, C
HOH), 2.54 (1H, s, O
H), 2.38–2.42 (m, 2H, C
H2), 2.04–2.25 (m, 7H, 2×
CH2 and
CH3), 1.68–1.75 (m, 2H, C
H2);
13C-NMR (CDCl
3): δ
C 170.9, 129.6, 128.6, 77.3, 72.1, 32.8, 30.0, 22.8, 22.8, 21.2. GC: Cromopack column t
R = 56.6 min HRMS: calcd for C
8H
14O
2 [M-CH
2CO]
+: 142.09938, Found: 142.0993 (0 ppm).
3.6. (1R,2R)-(Z)-2-Acetoxy-cyclooct-4-enyle Acetate (4a)
Compound
4a was obtained as a colorless oil.
![Molecules 19 09215 i001]()
+81.0° (
c 1.00 CHCl
3). ν
max (cm
−1): 3016, 2938, 2866, 1732, 1654, 1431, 1370, 1226, 1244, 1032, 978, 946, 735, 722;
1H-NMR (CDCl
3): δ
H 5.69–5.62 (2H, m, C
H = C
H), 5.05–5.08 (2H, m, 2×C
HOH), 2.35–2.41 (2H, m, C
H2), 2.12–2.18 (2H, m, C
H2), 2.01–2.04 (2H, m, C
H2), 1.96 (s, 6H, 2×C
H3), 1.73–1.76 (2H, m, C
H2).
13C-NMR (CDCl
3): δ
C 170.1, 128.6, 73.6, 29.9, 22.7, 20.9; [α]
D: +83.9° (c = 1.00 CHCl
3) GC Cromopack column t
R = 61.2 min.
3.7. (1S,2S)-(Z)-2-Acetoxy-cyclooct-4-enyle acetate (4b)
Compound
4b was obtained as a colorless oil;
![Molecules 19 09215 i001]()
−79.0° (
c 1.00 CHCl
3). ν
max (cm
−1): 3016, 2938, 2866, 1732, 1654, 1431, 1370, 1226, 1244, 1032, 978, 946, 735, 722;
1H-NMR (CDCl
3): δ
H 5.69–5.62 (2H, m, C
H = C
H), 5.05–5.08 (2H, m, 2×C
HOH), 2.35–2.41 (2H, m, C
H2), 2.12–2.18 (2H, m, C
H2), 2.01–2.04 (2H, m, C
H2), 1.96 (s, 6H, 2×C
H3), 1.73–1.76 (2H, m, C
H2).
13C-NMR (DCl
3): δ
C 170.1, 128.6, 73.6, 29.9, 22.7, 20.9; GC: Cromapack column t
R = 59.4 min; HRMS calcd for C
10H
16O
3 [M-CH
2CO]
+: 184.10994, Found: 184.1091 (4 ppm).
3.8. General Procedure for Enantioselective Acetylation of Racemic (Z)–Cyclooct-5-en-1,2-diol (2) Using Lipase
Under inert atmosphere, to a solution of racemic diol
2 (0.2 g, 1.41 mmol) solubilized in 2.5 mL of THF were added the vinyl acetate (1.3 mL, 14 mmol) and the appropriate immobilized lipase (50 mg). The mixture was stirred at the required temperature under classical heating or microwave irradiation (see
Table 1,
Table 2 and
Table 3). The mixture was filtrated, extracted with ethyl acetate (3 × 7 mL). The organic layers were washed with 3 mL of HCl 5%, 3 mL of sodium hydrogenocarbonate, brine, dried with magnesium sulfate and concentrated under reduced pressure. The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 60/40) to give the (
1R,
2R)-(
Z)
-1-hydroxy-cyclooct-4-enyl acetates
3a,
3b,
4b.
3.9. General Procedure for the Hydrolysis of (Z)-2-Acetoxy-cyclooct-4-enyle Acetate (4) by Candida Antarctica Lipase B
Under inert atmosphere, to a solution of racemic (
Z)
-2-acetoxycyclooct-4-enyl acetate
4 (0.2 g, 0.88 mmol) in 2.5 mL of phosphate buffer 0.1 M, pH = 7.0, was added the lipase (50 mg). The mixture was stirred at 50 °C under microwave irradiation (see
Table 4,
Table 5 and
Table 6). The mixture was filtrated, extracted with ethyl acetate (3 × 7 mL). The organic layers were washed with 3 mL of HCl 5%, 3 mL of sodium hydrogenocarbonate, brine, dried with magnesium sulfate and concentrated under reduced pressure. The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 60/40) to give
3a,
3b and
4a,
4b.
3.10. Diacetate Enrichment
The enriched (Z)-2-acetoxycyclooct-4-enyl compound 4a is solubilized in 2.5 mL of phosphate buffer 0.1 M, pH = 7.0. Candida antarctica lipase B (50 mg) was added and the mixture was stirred at 50 °C during 14 h by microwave irradiation (open vessel). The mixture was filtrated, extracted with ethyl acetate (3 × 7 mL), The combined organic layers were washed with 3 mL of HCl 5%, 3 mL of sodium hydrogenocarbonate, brine, dried with magnesium sulfate and concentrated under reduced pressure. The crude residue was purified by chromatography column (silica gel, petroleum ether/ethyl acetate 60/40) to give the (Z)-(1S,2S)-2-hydroxy-cyclooct-4-enyl acetate 3b in 49% overall yield (0.098 g, ee > 99%) and 4a in 51% overall yield (0.083 g, ee > 99%).
3.11. Influence of the Power of the Microwave for the Acetylation of (Z)–Cyclooct-5-en-1,2-diol (2)
The cryogenic fluid (Garlon 80®) of the Coolmate® was cooled by dry ice, and maintained at 7 °C. To a solution of racemic diol 2 (0.2 g, 1.406 mmol) solubilized in 2.5 mL of THF was added vinyl acetate (1.3 mL, 14 mmol) and Candida antarctica lipase (50 mg). The mixture was irradiated with an internal temperature set to 35 °C, leading to an irradiation power of 300 W. After 7 h of irradiation, the mixture was filtrated, extracted with ethyl acetate (3 × 7 mL). The organic layers were washed with 3 mL of HCl 5%, 3 mL of sodium hydrogenocarbonate, brine, dried with magnesium sulfate and concentrated under reduced pressure. The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 60/40) to give the (Z)-(1R,2R)-1-hydroxycyclooct-4-enyl acetate 3a in 42% yield (0.108 g, ee = 67%), (Z)-(1S,2S)-2-acetoxycyclooct-4-enyl acetate 4b in 2% yield (ee = 99%) and (Z)–cyclooct-5-en-1,2-diol in 51% yield (0.102 g, ee = 50%).
3.12. Synthesis of Diols by Saponification of Esters
Under inert atmosphere, to a solution of enantiopure (Z)-(1R,2R)-2-acetoxycyclooct-4-enyl acetate 4b (0.2 g, 17.3 mmol) or enantiopure monoacetate 3a (0.2 g, 12.0 mmol) in methanol (10 mL) was added potassium carbonate anhydrous (4 mg, 0.86 mmol). The mixture was stirred 8 h at 0 °C, and 10 mL of hydrochloric acid 1 M are added. The aqueous layer was extracted with ethyl acetate (3 × 8 mL), washed with a saturated solution of sodium hydrogenocarbonate (5 mL) and brine (5 mL). The organic layers were dried with magnesium sulfate, concentrated under reduced pressure.
3.13. (1R,2R)-(Z)-Cyclooct-5-ene-1,2-diol (2a)
The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 60/40) to give the (
Z)–(1
R,2
R)-cyclooct-5-ene-1,2-diol
2a (0.152 g, 99% yield) as a white solid;
![Molecules 19 09215 i001]()
−20.9° (
c 1,00 CHCl
3); ν
max (cm
−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719;
1H-NMR (CDCl
3): δ
H 5.55–5.59 (2H, m, C
H = C
H), 3.57–3.61 (m, 4H, 2×C
HO
H), 2.29–2.35 (2H, m, C
H2), 2.00–2.12 (2H, m, C
H2), 1.52–1.58 (2H, m, C
H2);
13C-NMR (CDCl
3): δ
c 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C
8H
14O
2 [M]
+: 142.09938, Found: 142.1001 (5 ppm). HPLC t
R = 11.9 min.
3.14. (1S,2S)-(Z)-Cyclooct-5-ene-1,2-diol (2b)
The crude residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 60/40) to give the (
Z)–(1
S,2
S)-cyclooct-5-en-1,2-diol
2b (0.153 g, 99% yield) as a white solid.
![Molecules 19 09215 i001]()
+18.2° (c = 1,00 CHCl
3)ν
max (cm
−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719;
1H-NMR (CDCl
3): δ
H 5.55–5.59 (2H, m, C
H = C
H), 3.57–3.61 (m, 4H, 2×C
HO
H), 2.29–2.35 (2H, m, C
H2), 2.00–2.12 (2H, m, C
H2), 1.52–1.58 (2H, m, C
H2);
13C-NMR (CDCl
3): δ
c 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C
8H
14O
2 [M]
+: 142.09938, Found: 142.1001 (5 ppm). HPLC t
R = 10.8 min.








 −3.0° (c 1.00 CHCl3). νmax (cm−1): 3449, 3012, 2936, 1717, 1654, 1430, 1235, 1030, 971, 933, 720; 1H-NMR (CDCl3): δH 5.59–5.69 (2H, m, CH = CH), 4.95 (1H dt, J = 8.8 Hz, 4.0Hz, CHOAc), 3.90 (1H, dt, J = 2.1 Hz, CHOH), 2.54 (1H, s, OH), 2.38–2.42 (m, 2H, CH2), 2.04–2.25 (m, 7H, 2×CH2 and CH3), 1.68–1.75 (m, 2H, CH2); 13C-NMR (CDCl3): δC 170.9, 129.6, 128.6, 77.3, 72.1, 32.8, 30.0, 22.8, 22.8, 21.2. GC: Cromopack column tR = 59.7 min HRMS: calcd for C8H14O2 [M-CH2CO]+: 142.09938, Found: 142.0993 (0 ppm).
−3.0° (c 1.00 CHCl3). νmax (cm−1): 3449, 3012, 2936, 1717, 1654, 1430, 1235, 1030, 971, 933, 720; 1H-NMR (CDCl3): δH 5.59–5.69 (2H, m, CH = CH), 4.95 (1H dt, J = 8.8 Hz, 4.0Hz, CHOAc), 3.90 (1H, dt, J = 2.1 Hz, CHOH), 2.54 (1H, s, OH), 2.38–2.42 (m, 2H, CH2), 2.04–2.25 (m, 7H, 2×CH2 and CH3), 1.68–1.75 (m, 2H, CH2); 13C-NMR (CDCl3): δC 170.9, 129.6, 128.6, 77.3, 72.1, 32.8, 30.0, 22.8, 22.8, 21.2. GC: Cromopack column tR = 59.7 min HRMS: calcd for C8H14O2 [M-CH2CO]+: 142.09938, Found: 142.0993 (0 ppm). +3.2° (c 1.00 CHCl3). νmax (cm−1): 3449, 3012, 2936, 1717, 1654, 1430, 1235, 1030, 971, 933, 720; 1H-NMR (CDCl3): δH 5.59–5.69 (2H, m, CH = CH), 4.95 (1H dt, J = 8.8 Hz, 4.0Hz, CHOAc), 3.90 (1H, dt, J = 2.1 Hz, CHOH), 2.54 (1H, s, OH), 2.38–2.42 (m, 2H, CH2), 2.04–2.25 (m, 7H, 2×CH2 and CH3), 1.68–1.75 (m, 2H, CH2); 13C-NMR (CDCl3): δC 170.9, 129.6, 128.6, 77.3, 72.1, 32.8, 30.0, 22.8, 22.8, 21.2. GC: Cromopack column tR = 56.6 min HRMS: calcd for C8H14O2 [M-CH2CO]+: 142.09938, Found: 142.0993 (0 ppm).
+3.2° (c 1.00 CHCl3). νmax (cm−1): 3449, 3012, 2936, 1717, 1654, 1430, 1235, 1030, 971, 933, 720; 1H-NMR (CDCl3): δH 5.59–5.69 (2H, m, CH = CH), 4.95 (1H dt, J = 8.8 Hz, 4.0Hz, CHOAc), 3.90 (1H, dt, J = 2.1 Hz, CHOH), 2.54 (1H, s, OH), 2.38–2.42 (m, 2H, CH2), 2.04–2.25 (m, 7H, 2×CH2 and CH3), 1.68–1.75 (m, 2H, CH2); 13C-NMR (CDCl3): δC 170.9, 129.6, 128.6, 77.3, 72.1, 32.8, 30.0, 22.8, 22.8, 21.2. GC: Cromopack column tR = 56.6 min HRMS: calcd for C8H14O2 [M-CH2CO]+: 142.09938, Found: 142.0993 (0 ppm). +81.0° (c 1.00 CHCl3). νmax (cm−1): 3016, 2938, 2866, 1732, 1654, 1431, 1370, 1226, 1244, 1032, 978, 946, 735, 722; 1H-NMR (CDCl3): δH 5.69–5.62 (2H, m, CH = CH), 5.05–5.08 (2H, m, 2×CHOH), 2.35–2.41 (2H, m, CH2), 2.12–2.18 (2H, m, CH2), 2.01–2.04 (2H, m, CH2), 1.96 (s, 6H, 2×CH3), 1.73–1.76 (2H, m, CH2). 13C-NMR (CDCl3): δC 170.1, 128.6, 73.6, 29.9, 22.7, 20.9; [α]D: +83.9° (c = 1.00 CHCl3) GC Cromopack column tR = 61.2 min.
+81.0° (c 1.00 CHCl3). νmax (cm−1): 3016, 2938, 2866, 1732, 1654, 1431, 1370, 1226, 1244, 1032, 978, 946, 735, 722; 1H-NMR (CDCl3): δH 5.69–5.62 (2H, m, CH = CH), 5.05–5.08 (2H, m, 2×CHOH), 2.35–2.41 (2H, m, CH2), 2.12–2.18 (2H, m, CH2), 2.01–2.04 (2H, m, CH2), 1.96 (s, 6H, 2×CH3), 1.73–1.76 (2H, m, CH2). 13C-NMR (CDCl3): δC 170.1, 128.6, 73.6, 29.9, 22.7, 20.9; [α]D: +83.9° (c = 1.00 CHCl3) GC Cromopack column tR = 61.2 min. −79.0° (c 1.00 CHCl3). νmax (cm−1): 3016, 2938, 2866, 1732, 1654, 1431, 1370, 1226, 1244, 1032, 978, 946, 735, 722; 1H-NMR (CDCl3): δH 5.69–5.62 (2H, m, CH = CH), 5.05–5.08 (2H, m, 2×CHOH), 2.35–2.41 (2H, m, CH2), 2.12–2.18 (2H, m, CH2), 2.01–2.04 (2H, m, CH2), 1.96 (s, 6H, 2×CH3), 1.73–1.76 (2H, m, CH2). 13C-NMR (DCl3): δC 170.1, 128.6, 73.6, 29.9, 22.7, 20.9; GC: Cromapack column tR = 59.4 min; HRMS calcd for C10H16O3 [M-CH2CO]+: 184.10994, Found: 184.1091 (4 ppm).
−79.0° (c 1.00 CHCl3). νmax (cm−1): 3016, 2938, 2866, 1732, 1654, 1431, 1370, 1226, 1244, 1032, 978, 946, 735, 722; 1H-NMR (CDCl3): δH 5.69–5.62 (2H, m, CH = CH), 5.05–5.08 (2H, m, 2×CHOH), 2.35–2.41 (2H, m, CH2), 2.12–2.18 (2H, m, CH2), 2.01–2.04 (2H, m, CH2), 1.96 (s, 6H, 2×CH3), 1.73–1.76 (2H, m, CH2). 13C-NMR (DCl3): δC 170.1, 128.6, 73.6, 29.9, 22.7, 20.9; GC: Cromapack column tR = 59.4 min; HRMS calcd for C10H16O3 [M-CH2CO]+: 184.10994, Found: 184.1091 (4 ppm). −20.9° (c 1,00 CHCl3); νmax (cm−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719; 1H-NMR (CDCl3): δH 5.55–5.59 (2H, m, CH = CH), 3.57–3.61 (m, 4H, 2×CHOH), 2.29–2.35 (2H, m, CH2), 2.00–2.12 (2H, m, CH2), 1.52–1.58 (2H, m, CH2); 13C-NMR (CDCl3): δc 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C8H14O2 [M]+: 142.09938, Found: 142.1001 (5 ppm). HPLC tR = 11.9 min.
−20.9° (c 1,00 CHCl3); νmax (cm−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719; 1H-NMR (CDCl3): δH 5.55–5.59 (2H, m, CH = CH), 3.57–3.61 (m, 4H, 2×CHOH), 2.29–2.35 (2H, m, CH2), 2.00–2.12 (2H, m, CH2), 1.52–1.58 (2H, m, CH2); 13C-NMR (CDCl3): δc 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C8H14O2 [M]+: 142.09938, Found: 142.1001 (5 ppm). HPLC tR = 11.9 min. +18.2° (c = 1,00 CHCl3)νmax (cm−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719; 1H-NMR (CDCl3): δH 5.55–5.59 (2H, m, CH = CH), 3.57–3.61 (m, 4H, 2×CHOH), 2.29–2.35 (2H, m, CH2), 2.00–2.12 (2H, m, CH2), 1.52–1.58 (2H, m, CH2); 13C-NMR (CDCl3): δc 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C8H14O2 [M]+: 142.09938, Found: 142.1001 (5 ppm). HPLC tR = 10.8 min.
+18.2° (c = 1,00 CHCl3)νmax (cm−1): 3362, 3014, 2964, 2861, 1651, 1427, 1429, 1400, 1271, 1202, 1010, 994, 976, 947, 868, 732, 719; 1H-NMR (CDCl3): δH 5.55–5.59 (2H, m, CH = CH), 3.57–3.61 (m, 4H, 2×CHOH), 2.29–2.35 (2H, m, CH2), 2.00–2.12 (2H, m, CH2), 1.52–1.58 (2H, m, CH2); 13C-NMR (CDCl3): δc 128.9, 73.8, 33.1, 22.6; HRMS: calculated for C8H14O2 [M]+: 142.09938, Found: 142.1001 (5 ppm). HPLC tR = 10.8 min.