N-Alkyl Carbazole Derivatives as New Tools for Alzheimer’s Disease: Preliminary Studies
Abstract
:1. Introduction


2. Results and Discussion
2.1. Molecular Docking Simulation

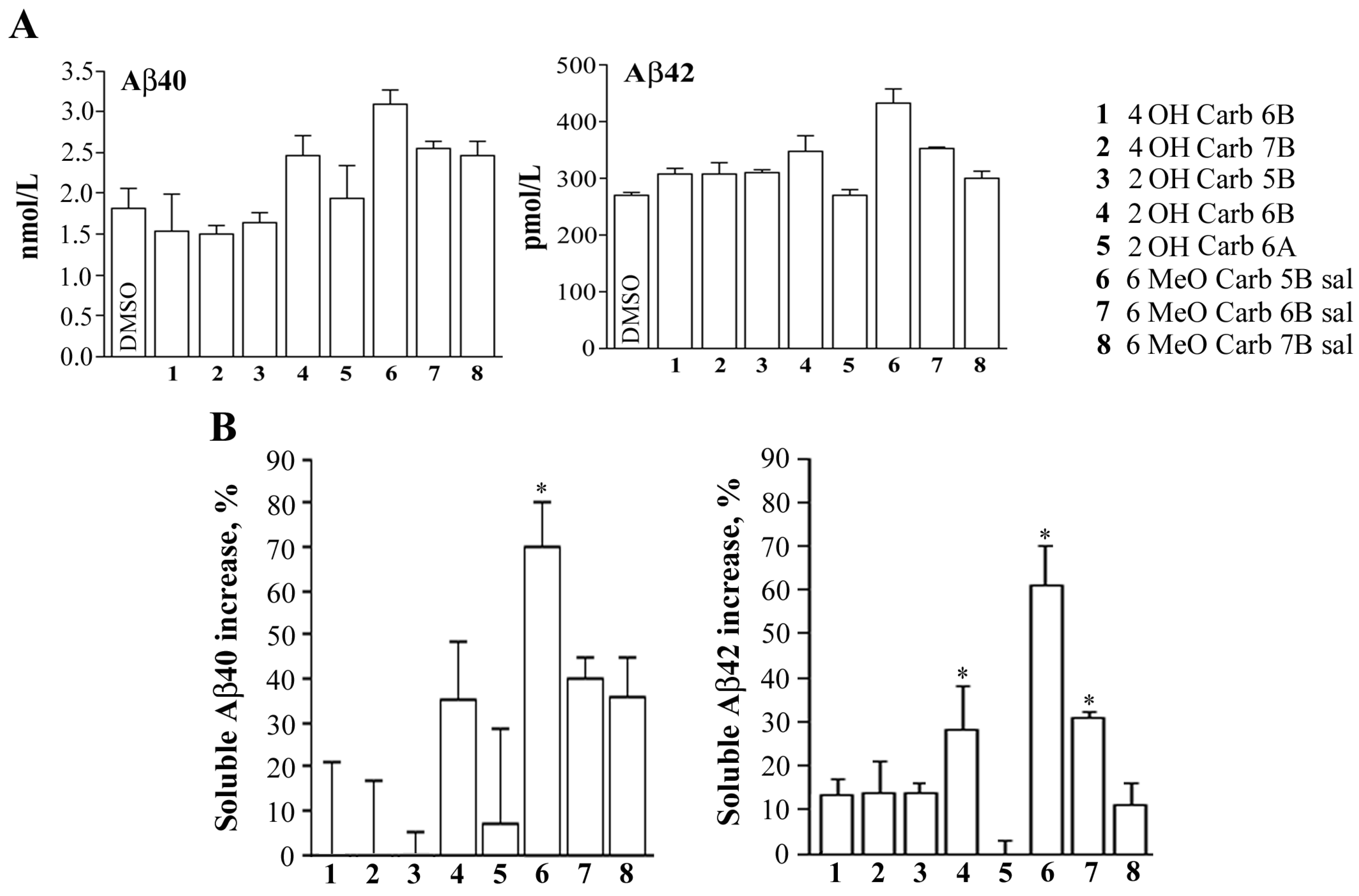
2.2. Biology
3. Experimental
3.1. Modeling
3.2. Cell Culture and Treatments
3.3. Aβ ELISA
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berchtold, N.C.; Cotman, C.W. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-roman period to the 1960s. Neurobiol. Aging 1998, 19, 173–189. [Google Scholar]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007, 3, 186–191. [Google Scholar]
- Haass, C.; Selkoe, D.J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 1993, 75, 1039–1042. [Google Scholar]
- De Strooper, B. Aph-1, pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron 2003, 38, 9–12. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Kihara, T.; Shimohama, S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. 2004, 64, 99–105. [Google Scholar]
- Jeppsson, F.; Eketjall, S.; Janson, J.; Karlstrom, S.; Gustavsson, S.; Olsson, L.L.; Radesater, A.C.; Ploeger, B.; Cebers, G.; Kolmodin, K.; et al. Discovery of azd3839, a potent and selective bace1 inhibitor clinical candidate for the treatment of alzheimer disease. J. Biol. Chem. 2012, 287, 41245–41257. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Munoz, E.; Salazar, J.R.; Yamaguchi, L.; Werner, E.; Alarcon, J.; Kubo, I. Inhibition of cholinesterase activity by extracts, fractions and compounds from calceolaria talcana and C. integrifolia (Calceolariaceae: Scrophulariaceae). Food Chem. Toxicol. 2013, 62, 919–926. [Google Scholar] [CrossRef]
- Bahrani, H.; Mohamad, J.; Paydar, M.J.; Rothan, H.A. Isolation and characterisation of acetylcholinesterase inhibitors from aquilaria subintegra for the treatment of Alzheimer’s disease (ad). Curr. Alzheimers Res. 2014, 11, 206–214. [Google Scholar] [CrossRef]
- Caruso, A.; Garofalo, A.; Grande, F.; Aiello, F.; Anzini, M.; Ortuso, F.; Alcaro, S.; Panno, A.; Saturnino, C.; Sinicropi, M.S. Synthesis and biological evaluation of 1,3-indandione derivatives as acetylcholinesterase inhibitors. Pharmacologyonline 2009, 1, 264–277. [Google Scholar]
- Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Synthesis, biological evaluation and molecular modeling study of novel tacrine-carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 21–30. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rossouw, J.E.; Siscovick, D.S.; Mouton, C.P.; Rifai, N.; Wallace, R.B.; Jackson, R.D.; Pettinger, M.B.; Ridker, P.M. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women’s health initiative observational study. JAMA 2002, 288, 980–987. [Google Scholar] [CrossRef]
- Saengkhae, C.; Salerno, M.; Ades, D.; Siove, A.; le Moyec, L.; Migonney, V.; Garnier-Suillerot, A. Ability of carbazole salts, inhibitors of alzheimer beta-amyloid fibril formation, to cross cellular membranes. Eur. J. Med. Chem. 2007, 559, 124–131. [Google Scholar]
- Howlett, D.R.; George, A.R.; Owen, D.E.; Ward, R.V.; Markwell, R.E. Common structural features determine the effectiveness of carvedilol, daunomycin and rolitetracycline as inhibitors of alzheimer beta-amyloid fibril formation. Biochem. J. 1999, 343, 419–423. [Google Scholar] [CrossRef]
- Caruso, A.; Sophie, A.; Voisin-Chiret ; Lancelot, J.C.; Sinicropi, M.S.; Garofalo, A.; Rault, S. Novel and efficient synthesis of 5,8-dimethyl-9h-carbazol-3-ol via a hydroxydeboronation reaction. Heterocycles 2007, 71, 2203–2210. [Google Scholar] [CrossRef]
- Caruso, A.; Voisin-Chiret, A.S.; Lancelot, J.C.; Sinicropi, M.S.; Garofalo, A.; Rault, S. Efficient and simple synthesis of 6-aryl-1,4-dimethyl-9h-carbazoles. Molecules 2008, 13, 1312–1320. [Google Scholar] [CrossRef]
- Sopkova-de Oliveira Santos, J.; Caruso, A.; Lohier, J.F.; Lancelot, J.C.; Rault, S. 9-Ethyl-1,4-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole and 6-bromo-9-ethyl-1,4-dimethyl-9h-carbazole. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2008, 64, o453–o455. [Google Scholar] [CrossRef]
- Caruso, A.; Lancelot, J.C.; El-Kashef, H.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Rault, S. A rapid and versatile synthesis of novel pyrimido[5,4-b]carbazoles. Tetrahedron 2009, 65, 10400–10405. [Google Scholar] [CrossRef]
- Lohier, J.F.; Caruso, A.; Sopková-de Oliveira Santos, J.; Lancelot, J.C.; Rault, S. tert-Butyl 6-bromo-1,4-dimethyl-9H-carbazole-9-carboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o1971–o1972. [Google Scholar] [CrossRef]
- Caruso, A.; Chimento, A.; El-Kashef, H.; Lancelot, J.C.; Panno, A.; Pezzi, V.; Saturnino, C.; Sinicropi, M.S.; Sirianni, R.; Rault, S. Antiproliferative activity of some 1,4-dimethylcarbazoles on cells that express estrogen receptors: Part I. J. Enzyme Inhib. Med. Chem. 2012, 27, 609–613. [Google Scholar] [CrossRef]
- Panno, A.; Sinicropi, M.S.; Caruso, A.; El-Kashef, H.; Lancelot, J.C.; Aubert, G.; Lesnard, A.; Cresteil, T.; Sylvain, R. New trimethoxybenzamides and trimethoxyphenylureas derived from dimethylcarbazole as cytotoxic agents. Part I. J. Heterocycl. Chem. 2014. [Google Scholar] [CrossRef]
- Caruso, A.; Lancelot, J.C.; El-Kashef, H.; Panno, A.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Lepailleur, A.; Rault, S. Four partners, three-step, one-pot reaction for a library of new 2-alkyl(dialkyl)amino-quinazolin-4(3H)-ones. J. Heterocycl. Chem. 2014. [Google Scholar] [CrossRef]
- Caruso, A.; Sinicropi, M.S.; Lancelot, J.C.; El-Kashef, H.; Saturnino, C.; Aubert, G.; Ballandonne, C.; Lesnard, A.; Cresteil, T.; Dallemagne, P.; et al. Synthesis and evaluation of cytotoxic activities of new guanidines derived from carbazoles. Bioorg. Med. Chem. Lett. 2014, 24, 467–472. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chen, M.H.; Li, M.; Luo, B.L.; Zhao, Y.; Huang, P.; Xue, F.T.; Rapposelli, S.; Pi, R.B.; Wen, S.J. Discovery of novel n-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Caruso, A.; Conforti, F.; Marrelli, M.; El Kashef, H.; Lancelot, J.C.; Rault, S.; Statti, G.A.; Menichini, F. Synthesis, inhibition of no production and antiproliferative activities of some indole derivatives. J. Enzyme Inhib. Med. Chem. 2009, 24, 1148–1153. [Google Scholar] [CrossRef]
- Bertini, S.; Asso, V.; Ghilardi, E.; Granchi, C.; Manera, C.; Minutolo, F.; Saccomanni, G.; Bortolato, A.; Mason, J.; Moro, S.; et al. Carbazole-containing arylcarboxamides as bace1 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 6657–6661. [Google Scholar] [CrossRef]
- Fang, L.; Fang, X.; Gou, S.; Lupp, A.; Lenhardt, I.; Sun, Y.; Huang, Z.; Chen, Y.; Zhang, Y.; Fleck, C. Design, synthesis and biological evaluation of d-ring opened galantamine analogs as multifunctional anti-alzheimer agents. Eur. J. Med. Chem. 2014, 76, 376–386. [Google Scholar] [CrossRef]
- Saturnino, C.; Palladino, C.; Napoli, M.; Sinicropi, M.S.; Botta, A.; Sala, M.; Carcereri de Prati, A.; Novellino, E.; Suzuki, H. Synthesis and biological evaluation of new n-alkylcarbazole derivatives as stat3 inhibitors: Preliminary study. Eur. J. Med. Chem. 2013, 60, 112–119. [Google Scholar] [CrossRef]
- Crescenzi, O.; Tomaselli, S.; Guerrini, R.; Salvadori, S.; D’Ursi, A.M.; Temussi, P.A.; Picone, D. Solution structure of the alzheimer amyloid beta-peptide (1–42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur. J. Biochem. 2002, 269, 5642–5648. [Google Scholar] [CrossRef]
- Luhrs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Dobeli, H.; Schubert, D.; Riek, R. 3D structure of Alzheimer’s amyloid-beta(1–42) fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef]
- Brunger, A.T.; Adams, P.D.; Clore, G.M.; DeLano, W.L.; Gros, P.; Grosse-Kunstleve, R.W.; Jiang, J.S.; Kuszewski, J.; Nilges, M.; Pannu, N.S.; et al. Crystallography & nmr system: A new software suite for macromolecular structure determination. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 905–921. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Iacopetta, D.; Madeo, M.; Tasco, G.; Carrisi, C.; Curcio, R.; Martello, E.; Casadio, R.; Capobianco, L.; Dolce, V. A novel subfamily of mitochondrial dicarboxylate carriers from drosophila melanogaster: Biochemical and computational studies. Biochim. Biophys. Acta 2011, 1807, 251–261. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules (vol 117, pg 5179, 1995). J. Am. Chem. Soc. 1996, 118, 2309–2309. [Google Scholar]
- Canepa, E.; Borghi, R.; Vina, J.; Traverso, N.; Gambini, J.; Domenicotti, C.; Marinari, U.M.; Poli, G.; Pronzato, M.A.; Ricciarelli, R. Cholesterol and amyloid-beta: Evidence for a cross-talk between astrocytes and neuronal cells. J. Alzheimers Dis. 2011, 25, 645–653. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saturnino, C.; Iacopetta, D.; Sinicropi, M.S.; Rosano, C.; Caruso, A.; Caporale, A.; Marra, N.; Marengo, B.; Pronzato, M.A.; Parisi, O.I.; et al. N-Alkyl Carbazole Derivatives as New Tools for Alzheimer’s Disease: Preliminary Studies. Molecules 2014, 19, 9307-9317. https://doi.org/10.3390/molecules19079307
Saturnino C, Iacopetta D, Sinicropi MS, Rosano C, Caruso A, Caporale A, Marra N, Marengo B, Pronzato MA, Parisi OI, et al. N-Alkyl Carbazole Derivatives as New Tools for Alzheimer’s Disease: Preliminary Studies. Molecules. 2014; 19(7):9307-9317. https://doi.org/10.3390/molecules19079307
Chicago/Turabian StyleSaturnino, Carmela, Domenico Iacopetta, Maria Stefania Sinicropi, Camillo Rosano, Anna Caruso, Angelamaria Caporale, Nancy Marra, Barbara Marengo, Maria Adelaide Pronzato, Ortensia Ilaria Parisi, and et al. 2014. "N-Alkyl Carbazole Derivatives as New Tools for Alzheimer’s Disease: Preliminary Studies" Molecules 19, no. 7: 9307-9317. https://doi.org/10.3390/molecules19079307






