Self-Assembled Micelles Composed of Doxorubicin Conjugated Y-Shaped PEG-Poly(glutamic acid)2 Copolymers via Hydrazone Linkers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Polymers



| Copolymer a | Feed molar ratio of [I]/[N] b | Mn of copolymer c (Da) | DP d | Initiator efficiency e | Polydispersity f (MW/Mn) |
|---|---|---|---|---|---|
| PEG5k-PBLG5.7k | 1/30 | 10694 | 26 | 0.87 | 1.07 |
| PEG5k-PBLG13.6k | 1/70 | 18578 | 62 | 0.89 | 1.19 |
| PEG5k-(PBLG5.7k)2 | 1/65 | 16388 | 52 | 0.80 | 1.11 |
| PEG5k-(PBLG6.8k)2 | 1/75 | 18578 | 62 | 0.83 | 1.11 |

| Sample | Polymer | Size (nm) | Polydispersity index | DLC (%) | CAC (mg/L) |
|---|---|---|---|---|---|
| PEG-PGDI | PEG-P(Glu)26-DOX | 149.9 ± 3.7 | 0.081 ± 0.023 | 9.92 ± 0.25 | 9.0 |
| PEG-PGDII | PEG-P(Glu)62-DOX | 231.4 ± 8.3 | 0.229 ± 0.061 | 18.8 ± 0.18 | 4.2 |
| PEG-PG2DI | PEG-P(Glu26)2-DOX | 141.3 ± 5.2 | 0.222 ± 0.042 | 16.2 ± 0.12 | 5.4 |
| PEG-PG2DII | PEG-P(Glu31)2-DOX | 165.6 ± 2.4 | 0.116 ± 0.035 | 18.2 ± 0.45 | 3.8 |
2.2. Preparation of DOX-Conjugated Polymer Micelles

2.3. In Vitro Drug Release Studies
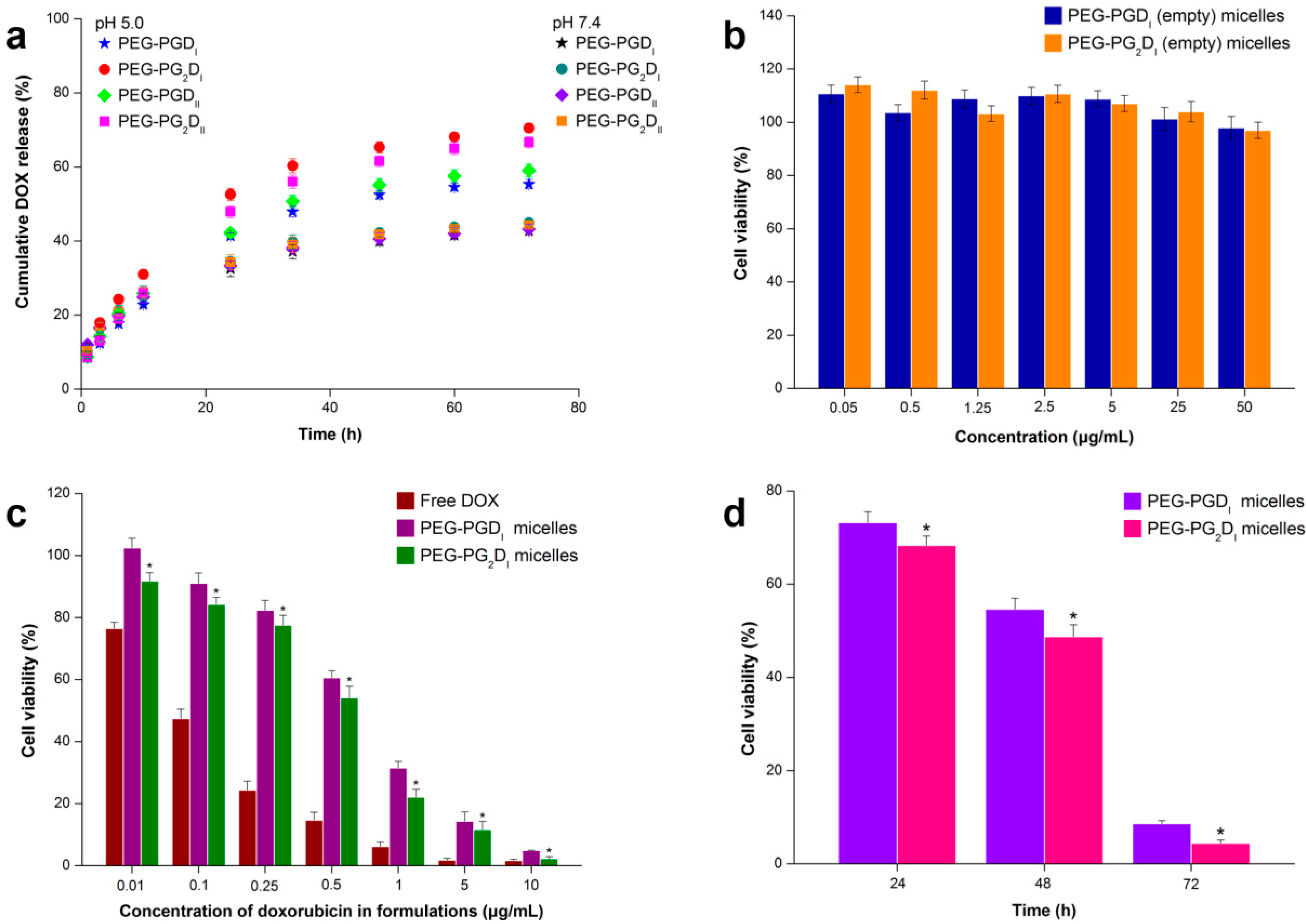
2.4. Cell Cytotoxicity Studies
2.5. Cellular Uptake Study

3. Experimental Section
3.1. Materials
3.2. Synthesis of γ-benzyl-L-glutamate-N-carboxy anhydride
3.3. Synthesis of Methoxyl Poly (ethylene glycol)-Poly (γ-benzyl-l-glutamate) Diblock and AB2 Y-Shaped 3-Miktoarm Star-Block Copolymers
3.4. Synthesis of Methoxyl Poly (ethylene glycol)-Poly (glutamate-hydrazone-doxorubicin)2 Polymer
3.5. Material Characterization
3.6. Preparation and Characterization of the pH-Sensitive Polymeric Micelles
3.7. In Vitro Release Studies
3.8. In Vitro Cytotoxicity Assay

3.9. Confocal Analysis
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Kulhari, H.; Pooja, D.; Singh, M.K.; Chauhan, A.S. Optimization of carboxylate-terminated poly (amidoamine) dendrimer-mediated cisplatin formulation. Drug Dev. Ind. Pharm. 2013, 0, 1–7. [Google Scholar]
- Zhou, Z.; D’Emanuele, A.; Attwood, D. Solubility enhancement of paclitaxel using a linear-dendritic block copolymer. Int. J. Pharm. 2013, 1, 173–179. [Google Scholar]
- Jeong, J.H.; Byun, Y.; Park, T.G. Synthesis and characterization of poly (l-lysine)-g-poly (d, l-lactic-co-glycolic acid) biodegradable micelles. J. Biomater. Sci. Polym. Ed. 2003, 14, 1–11. [Google Scholar]
- Sun, Y.; Yan, X.; Yuan, T.; Liang, J.; Fan, Y.; Gu, Z.; Zhang, X. Disassemblable micelles based on reduction-degradable amphiphilic graft copolymers for intracellular delivery of doxorubicin. Biomaterials 2010, 31, 7124–7131. [Google Scholar]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar]
- Gu, P.F.; Xu, H.; Sui, B.W.; Gou, J.X.; Meng, L.K.; Sun, F.; Wang, X.J.; Qi, N.; Zhang, Y.; He, H.B. Polymeric micelles based on poly(ethylene glycol) block poly(racemic amino acids) hybrid polypeptides: Conformation-facilitated drug-loading behavior and potential application as effective anticancer drug carriers . Int. J. Nanomed. 2012, 7, 109–122. [Google Scholar]
- Li, H.; Diao, M.; Zhang, S.; Wang, K.; Xue, C. Novel polymeric micelles of ab2 type-methoxy-poly(ethylene glycol)-b-poly(γ-benzyl-l-glutamate)2 copolymers as tamoxifen carriers. J. Nanosci. Nanotech. 2009, 9, 4805–4811. [Google Scholar]
- Yin, H.; Kang, S.W.; Bae, Y.H. Polymersome formation from ab2 type 3-miktoarm star copolymers. Macromolecules 2009, 42, 7456–7464. [Google Scholar]
- Sun, J.; Chen, X.; Guo, J.; Shi, Q.; Xie, Z.; Jing, X. Synthesis and self-assembly of a novel y-shaped copolymer with a helical polypeptide arm. Polymer 2009, 50, 455–461. [Google Scholar]
- Rao, J.; Zhang, Y.; Zhang, J.; Liu, S. Facile preparation of well-defined ab2 y-shaped miktoarm star polypeptide copolymer via the combination of ring-opening polymerization and click chemistry. Biomacromolecules 2008, 9, 2586–2593. [Google Scholar]
- Liu, H.; Xu, J.; Jiang, J.; Yin, J.; Narain, R.; Cai, Y.; Liu, S. Syntheses and micellar properties of well-defined amphiphilic ab2 and a2b y-shaped miktoarm star copolymers of ε-caprolactone and 2-(dimethylamino)ethyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1446–1462. [Google Scholar]
- Yin, H.; Kang, H.C.; Huh, K.M.; Bae, Y.H. Biocompatible, pH-sensitive ab2 miktoarm polymer-based polymersomes: Preparation, characterization, and acidic pH-activated nanostructural transformation. J. Mater. Chem. 2012, 22, 19168–19178. [Google Scholar] [CrossRef]
- Li, Z.; Kesselman, E.; Talmon, Y.; Hillmyer, M.A.; Lodge, T.P. Multicompartment micelles from abc miktoarm stars in water. Science 2004, 306, 98–101. [Google Scholar]
- Li, Z.; Hillmyer, M.A.; Lodge, T.P. Laterally nanostructured vesicles, polygonal bilayer sheets, and segmented wormlike micelle. Nano Lett. 2006, 6, 1245–1249. [Google Scholar]
- Soliman, G.M.; Sharma, A.; Maysinger, D.; Kakkar, A. Dendrimers and miktoarm polymers based multivalent nanocarriers for efficient and targeted drug delivery. Chem. Commun. 2011, 47, 9572–9587. [Google Scholar]
- Li, Y.Y.; Zhang, X.Z.; Cheng, H.; Kim, G.C.; Cheng, S.X.; Zhuo, R.X. Novel stimuli-responsive micelle self-assembled from y-shaped p (ua-y-nipaam) copolymer for drug delivery. Biomacromolecules 2006, 7, 2956–2960. [Google Scholar]
- Riley, T.; Govender, T.; Stolnik, S.; Xiong, C.; Garnett, M.; Illum, L.; Davis, S. Colloidal stability and drug incorporation aspects of micellar-like pla–peg nanoparticles. Colloids Surf. B Biointerf. 1999, 16, 147–159. [Google Scholar]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B. Biointerf. 1999, 16, 3–27. [Google Scholar]
- Barenholz, Y.C. Doxil®—the first fda-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar]
- Upadhyay, K.K.; Bhatt, A.N.; Mishra, A.K.; Dwarakanath, B.S.; Jain, S.; Schatz, C.; Le Meins, J.F.; Farooque, A.; Chandraiah, G.; Jain, A.K. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly (γ-benzyl-l-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010, 31, 2882–2892. [Google Scholar]
- Lv, S.; Li, M.; Tang, Z.; Song, W.; Sun, H.; Liu, H.; Chen, X. Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy. Acta Biomater. 2013, 9, 9330–9342. [Google Scholar]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Block copolymer micelles for drug delivery: Loading and release of doxorubicin. J. Control. Release 1997, 48, 195–201. [Google Scholar]
- Yang, X.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Matson, V.Z.; Steeber, D.A.; Gong, S. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive mr imaging. ACS Nano 2010, 4, 6805–6817. [Google Scholar]
- Lee, H.J.; Bae, Y. Pharmaceutical differences between block copolymer self-assembled and cross-linked nanoassemblies as carriers for tunable drug release. Pharm. Res. 2013, 30, 478–488. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. JACS 1977, 99, 2039–2044. [Google Scholar]
- Yang, J.; Zhang, D.; Jiang, S.; Yang, J.; Nie, J. Synthesis of y-shaped poly(solketal acrylate)-containing block copolymers and study on the thermoresponsive behavior for micellar aggregates. J. Colloid Interface Sci. 2010, 352, 405–414. [Google Scholar]
- Maglio, G.; Nicodemi, F.; Conte, C.; Palumbo, R.; Tirino, P.; Panza, E.; Ianaro, A.; Ungaro, F.; Quaglia, F. Nanocapsules based on linear and y-shaped 3-miktoarm star-block peo-pcl copolymers as sustained delivery system for hydrophilic molecules. Biomacromolecules 2011, 12, 4221–4229. [Google Scholar]
- Diao, M. Preparation and Properties of Tamoxifen-Loaded Micelles of Poly(ethylene glycol)-Poly(γ-benzyl-l-glutamate)2 Copolymer. Master Thesis, Hunan University, Changsha, China, 2008. [Google Scholar]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.; Miyazono, K.; Uesaka, M. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotech. 2011, 6, 815–823. [Google Scholar]
- Nie, S.; Hsiao, W.W.; Pan, W.; Yang, Z. Thermoreversible pluronic® f127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular ph-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconj. Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ha, J.C.; Lee, Y.M. Poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide)/poly(ε caprolactone)(PCL) amphiphilic block copolymeric nanospheres: II. Thermo-responsive drug release behaviors. J. Control. Release 2000, 65, 345–358. [Google Scholar] [CrossRef]
- Zhao, Y.; Alakhova, D.Y.; Kim, J.O.; Bronich, T.K.; Kabanov, A.V. A simple way to enhance doxil® therapy: Drug release from liposomes at the tumor site by amphiphilic block copolymer. J. Control. Release 2013, 168, 61–69. [Google Scholar]
- Tsukioka, Y.; Matsumura, Y.; Hamaguchi, T.; Koike, H.; Moriyasu, F.; Kakizoe, T. Pharmaceutical and biomedical differences between micellar doxorubicin (nk911) and liposomal doxorubicin (doxil). Cancer Sci. 2002, 93, 1145–1153. [Google Scholar]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699–6707. [Google Scholar]
- Watson, P.; Jones, A.T.; Stephens, D.J. Intracellular trafficking pathways and drug delivery: Fluorescence imaging of living and fixed cells. Adv. Drug Del. Rev. 2005, 57, 43–61. [Google Scholar]
- Liu, S.Q.; Wiradharma, N.; Gao, S.J.; Tong, Y.W.; Yang, Y.Y. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomaterials 2007, 28, 1423–1433. [Google Scholar]
- Zhao, H.; Yung, L.Y.L. Selectivity of folate conjugated polymer micelles against different tumor cells. Int. J. Pharm. 2008, 349, 256–268. [Google Scholar]
- Daly, W.H.; Poché, D. The preparation of N-carboxyanhydrides of α-amino acids using bis (trichloromethyl) carbonate. Tetrahedron Lett. 1988, 29, 5859–5862. [Google Scholar] [CrossRef]
- Shan, Y.; Qin, Y.; Chuan, Y.; Li, H.; Yuan, M. The synthesis and characterization of hydroxyapatite-β-alanine modified by grafting polymerization of γ-benzyl-l-glutamate-N-carboxyanhydride. Molecules 2013, 18, 13979–13991. [Google Scholar]
- Meng, Y.; Song, Y.; Yan, Z.; Xia, Y. Synthesis and in vitro cytotoxicity of novel ursolic acid derivatives. Molecules 2010, 15, 4033–4040. [Google Scholar] [CrossRef]
- Cao, J.; Zhai, S.; Li, C.; He, B.; Lai, Y.; Chen, Y.; Luo, X.; Gu, Z. Novel pH-sensitive micelles generated by star-shape copolymers containing zwitterionic sulfobetaine for efficient cellular internalization. J. Biomed. Nanotech 2013, 9, 1847–1861. [Google Scholar]
- Samples Availability: Not Available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sui, B.; Xu, H.; Jin, J.; Gou, J.; Liu, J.; Tang, X.; Zhang, Y.; Xu, J.; Zhang, H.; Jin, X. Self-Assembled Micelles Composed of Doxorubicin Conjugated Y-Shaped PEG-Poly(glutamic acid)2 Copolymers via Hydrazone Linkers. Molecules 2014, 19, 11915-11932. https://doi.org/10.3390/molecules190811915
Sui B, Xu H, Jin J, Gou J, Liu J, Tang X, Zhang Y, Xu J, Zhang H, Jin X. Self-Assembled Micelles Composed of Doxorubicin Conjugated Y-Shaped PEG-Poly(glutamic acid)2 Copolymers via Hydrazone Linkers. Molecules. 2014; 19(8):11915-11932. https://doi.org/10.3390/molecules190811915
Chicago/Turabian StyleSui, Bowen, Hui Xu, Jian Jin, Jingxin Gou, Jingshuo Liu, Xing Tang, Yu Zhang, Jinghua Xu, Hongfeng Zhang, and Xiangqun Jin. 2014. "Self-Assembled Micelles Composed of Doxorubicin Conjugated Y-Shaped PEG-Poly(glutamic acid)2 Copolymers via Hydrazone Linkers" Molecules 19, no. 8: 11915-11932. https://doi.org/10.3390/molecules190811915




