Bioevaluation of Novel Anti-Biofilm Coatings Based on PVP/Fe3O4 Nanostructures and 2-((4-Ethylphenoxy)methyl)-N- (arylcarbamothioyl)benzamides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of New 2-((4-Ethylphenoxy)methyl)-N-(arylcarbamothioyl)benzamides 1a–c

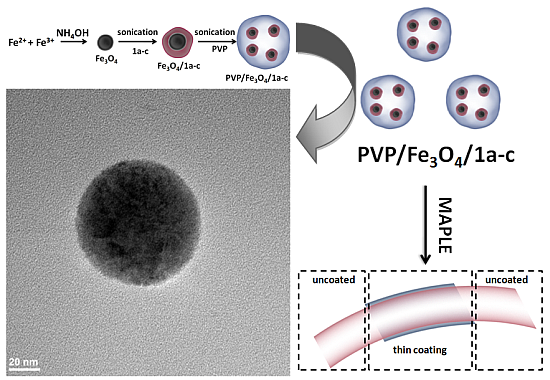
2.2. PVP/Fe3O4/1a–c Characterization

2.3. Thin Films Characterization
2.3.1. IR





2.3.2. SEM

2.4. Biological Assays
2.4.1. Viability and Cell Proliferation


2.4.2. In Vitro Microbial Biofilm Development



3. Experimental
3.1. Materials
3.2. Preparation of Thiourea Functionalized Magnetite Nanoparticles

3.3. MAPLE Thin Coating Deposition

3.4. Synthesis of 2-((4-Ethylphenoxy)methyl)-N-(arylcarbamothioyl)benzamides 1a–c
3.5. Characterization
3.5.1. X-ray Diffraction
3.5.2. Infrared Microscopy
3.5.3. Scanning Electron Microscopy
3.5.4. TransmissionElectronMicroscopy
3.6. Biological Characterization
3.6.1. Viability and Cell Proliferation
3.6.1.1. Cell Culture
3.6.1.2. Cell Viability
3.6.2. In Vitro Microbial Biofilm Development
3.6.3. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saeed, A.; Flörke, U.; Erben, M.F. A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J. Sulfur Chem. 2013, 34, 318–355. [Google Scholar]
- Struga, M.; Kossakowski, J.; Koziol, A.E.; Kedzierska, E.; Fidecka, S.; La Colla, P.; Ibba, C.; Collu, G.; Sanna, G.; Secci, B.; et al. Synthesis, pharmacological and antiviral activity of 1,3-thiazepine derivatives. Eur. J. Med. Chem. 2009, 44, 4960–4969. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N.; Jones, G.P.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Zhang, T.; Zhu, H.; Qian, H.; Zhang, H.; Huang, W.; Gust, R. Design and synthesis of thiourea derivatives containing a benzo[5,6]cyclohepta[1,2-b]pyridine moiety as potential antitumor and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2012, 22, 2701–2704. [Google Scholar] [CrossRef]
- Vega-Pérez, J.M.; Periñán, I.; Argandoña, M.; Vega-Holm, M.; Palo-Nieto, C.; Burgos-Morón, E.; López-Lázaro, M.; Vargas, C.; Nieto, J.J.; Iglesias-Guerra, F. Isoprenyl-thiourea and urea derivatives as new farnesyl diphosphate analogues: Synthesis and in vitro antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2012, 58, 591–612. [Google Scholar] [CrossRef]
- Saeed, A.; Abbas, N.; Ashraf, Z.; Bolte, M. Synthesis, characterization and antibacterial activity of new 1,2- and 1,4-bis(N'-substituted thioureido)benzene derivatives. S. Afr. J. Chem. 2013, 66, 273–278. [Google Scholar]
- Masereel, B.; Wouters, J.; Pochet, L.; Lambert, D. Design, synthesis and anticonvulsant activity of 1-(pyrid-3-ylsulfonamido)-2-nitroethylenes. J. Med. Chem. 1998, 41, 3239–3244. [Google Scholar] [CrossRef]
- Walchshofer, N.; Delabre-Defayolle, I.; Paris, J.; Petavy, A.F. In vivo morphological damage induced by a new benzimidazole prodrug in Echinococcus multilocularis metacestodes. J. Pharm. Sci. 1990, 79, 606–608. [Google Scholar] [CrossRef]
- Loto, R.T.; Loto, C.A.; Popoola, A.P.I. Corrosion inhibition of thiourea and thiadiazole derivatives: A Review. J.Mater. Environ. Sci. 2012, 3, 885–894. [Google Scholar]
- Kachhadia, V.V.; Patel, M.R.; Joshi, H.S. Heterocyclic systems containing S/N regioselective nucleophilic competition: facile synthesis, antitubercular and antimicrobial activity of thiohydantoins and iminothiazolidinone containing the benzo[b]thiophene moiety. J. Serb. Chem. Soc. 2005, 70, 153–161. [Google Scholar] [CrossRef]
- Saeed, A.; Erben, M.F.; Bolte, M. Synthesis, structural and vibrational properties of 1-(adamantane-1-carbonyl)-3-halophenyl thioureas. Spectrochim. ActaA 2013, 102, 408–413. [Google Scholar] [CrossRef]
- Kotb, E.R.; Anwar, M.M.; Abbas, H.A.; Abd El-Moez, S.I. A concise synthesis and antimicrobial activity of a novel series of naphthylpyridine-3-carbonitrile compounds. Acta Pol. Pharm. 2013, 70, 667–679. [Google Scholar]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 29, 1–22. [Google Scholar]
- Ladj, R.; Bitar, A.; Eissa, M.M.; Fessi, H.; Mugnier, Y.; Le Dantec, R.; Elaissari, A. Polymer encapsulation of inorganic nanoparticles for biomedical applications. Int. J. Pharm. 2013, 458, 230–241. [Google Scholar]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar]
- Mazor, L.P.; Dakwar, G.R.; Popov, M.; Kolusheva, S.; Shames, A.; Linder, C.; Greenberg, S.; Heldman, E.; Stepensky, D.; Jelinek, R. Bolaamphiphilic vesicles encapsulating iron oxide nanoparticles: New vehicles for magnetically targeted drug delivery. Int. J. Pharm. 2013, 450, 241–249. [Google Scholar]
- Filippousi, M.; Papadimitriou, S.; Bikiaris, D.N.; Pavlidou, E.; Angelakeris, M.; Zamboulis, D.; Tian, H.; van Tendeloo, G. Novel core–shell magnetic nanoparticles for Taxol encapsulation in biodegradable and biocompatible block copolymers: Preparation, characterization and release properties. Int. J. Pharm. 2013, 448, 221–230. [Google Scholar]
- David, S.; Marchais, H.; Hervé-Aubert, K.; Bedin, D.; Garin, A.S.; Hoinard, C.; Chourpa, I. Use of experimental design methodology for the development of new magnetic siRNA nanovectors (MSN). Int. J. Pharm. 2013, 454, 660–667. [Google Scholar]
- Kim, M.H.; Park, D.H.; Yang, J.H.; Choy, Y.B.; Choy, J.H. Drug-inorganic-polymer nanohybrid for transdermal delivery. Int. J. Pharm. 2013, 444, 120–127. [Google Scholar]
- Ahmad, H. Magnetic Polyaniline Composites: Recent Developments in preparation, properties and applications. J. Colloid Sci. Biot. 2013, 2, 155–170. [Google Scholar]
- Medeiros, S.F.; Lara, B.R.; Oliveira, P.F.M.; Moraes, R.M.; Alves, G.M.; Elaissari, A.; Santos, A.M. Stimuli-Responsive and Biocompatible Poly(N-vinylcaprolactam-co-acrylic acid)-coated iron oxide nanoparticles by nanoprecipitation technique. J. Colloid Sci. Biot. 2013, 2, 180–194. [Google Scholar]
- Roveimiab, Z.; Mahdavian, A.R.; Biazar, E.; Heidari, K.S. Preparation of magnetic chitosan nanocomposite particles and their susceptibility for cellular separation applications. J. Colloid Sci. Biot. 2012, 1, 82–88. [Google Scholar]
- Mary, Y.S.; Jojo, P.J.; Panicker, C.Y.; van Alsenoy, C.; Ataei, S.; Yildiz, I. Quantum mechanical and spectroscopic (FT-IR, FT-Raman, 1H NMR and UV) investigations of 2-(phenoxymethyl)benzimidazole. Spectrochim. Acta A 2014, 125, 12–24. [Google Scholar] [CrossRef]
- Limban, C.; Vasile, A.; Chirita, I.C.; Nitulescu, G.M.; Caproiu, M.T. Synthesis of new thiourea scaffold compounds. Rev. Chim.-Bucharest 2011, 62, 685–688. [Google Scholar]
- Byler, D.M.; Wilson, R.M.; Randall, C.S.; Sokoloski, T.D. Second derivative infrared spectroscopy as a nondestructive tool to assess the purity and structural integrity of proteins. Pharm. Res. 1995, 12, 446–450. [Google Scholar]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Lazar, V.; Chifiriuc, M.C. Mechanisms and experimental models for the assessment of biofilms phenotypic resistance/tolerance. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2011; pp. 906–911. [Google Scholar]
- Lazãr, V.; Chifiriuc, M.C. Medical significance and new therapeutical strategies for biofilm associated infections. Rom. Arch. Microbiol. Immunol. 2010, 69, 121–127. [Google Scholar]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Laz, R.V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Anghel, I.; Limban, C.; Grumezescu, A.M.; Anghel, A.G.; Bleotu, C.; Chifiriuc, M.C. In vitro evaluation of anti-pathogenic surface coating nanofluid, obtained by combining Fe3O4/C12nanostructures and 2-((4-ethylphenoxy)methyl)-N-(substituted-phenylcarbamothioyl)-benzamides. Nanoscale Res. Lett. 2012, 7, 513. [Google Scholar] [CrossRef]
- Anghel, I.; Grumezescu, A.M.; Andronescu, E.; Anghel, A.G.; Ficai, A.; Saviuc, C.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. Magnetite nanoparticles for functionalized textile dressing to prevent fungal biofilms development. Nanoscale Res. Lett. 2012, 7, 501. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Chifiriuc, M.C.; Saviuc, C.; Grumezescu, V.; Hristu, R.; Mihaiescu, D.E.; Stanciu, G.A.; Andronescu, E. Hybrid Nanomaterial for Stabilizing the Antibiofilm Activity of Eugenia carryophyllata Essential Oil. IEEE T. Nanobiosci. 2012, 11, 360–365. [Google Scholar] [CrossRef]
- Romberg, B.; Hennink, W.E.; Storm, G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008, 25, 55–71. [Google Scholar] [CrossRef]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, C.M.; Radulescu, R. Magnetite-usnic acid nanostructured bioactive material with antimicrobial activity. Rom. J. Mater. 2013, 43, 402–407. [Google Scholar] [CrossRef]
- Galateanu, B.; Dimonie, D.; Vasile, E.; Nae, S.; Cimpean, A.; Costache, M. Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotech. 2012, 12, 35. [Google Scholar] [CrossRef]
- Saviuc, C.; Grumezescu, A.M.; Oprea, E.A.; Radulescu, V.; Dascalu, L.; Chifiriuc, C.; Bucur, M.; Banu, O.; Lazar, V. Antifungal activity of some vegetal extracts on Candida biofilms developed on inert substratum. Bioint. Res. Appl. Chem. 2011, 1, 15–23. [Google Scholar]
- Saviuc, C.; Grumezescu, A.M.; Holban, A.; Chifiriuc, C.; Mihaiescu, D.; Lazar, V. Hybrid nanostructurated material for biomedical applications. Bioint. Res. Appl. Chem. 2011, 1, 64–71. [Google Scholar]
- Sample Availability: Samples of the compounds PVP/Fe3O4/1a–c are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Limban, C.; Missir, A.V.; Grumezescu, A.M.; Oprea, A.E.; Grumezescu, V.; Vasile, B.Ș.; Socol, G.; Trușcă, R.; Caproiu, M.T.; Chifiriuc, M.C.; et al. Bioevaluation of Novel Anti-Biofilm Coatings Based on PVP/Fe3O4 Nanostructures and 2-((4-Ethylphenoxy)methyl)-N- (arylcarbamothioyl)benzamides. Molecules 2014, 19, 12011-12030. https://doi.org/10.3390/molecules190812011
Limban C, Missir AV, Grumezescu AM, Oprea AE, Grumezescu V, Vasile BȘ, Socol G, Trușcă R, Caproiu MT, Chifiriuc MC, et al. Bioevaluation of Novel Anti-Biofilm Coatings Based on PVP/Fe3O4 Nanostructures and 2-((4-Ethylphenoxy)methyl)-N- (arylcarbamothioyl)benzamides. Molecules. 2014; 19(8):12011-12030. https://doi.org/10.3390/molecules190812011
Chicago/Turabian StyleLimban, Carmen, Alexandru Vasile Missir, Alexandru Mihai Grumezescu, Alexandra Elena Oprea, Valentina Grumezescu, Bogdan Ștefan Vasile, Gabriel Socol, Roxana Trușcă, Miron Teodor Caproiu, Mariana Carmen Chifiriuc, and et al. 2014. "Bioevaluation of Novel Anti-Biofilm Coatings Based on PVP/Fe3O4 Nanostructures and 2-((4-Ethylphenoxy)methyl)-N- (arylcarbamothioyl)benzamides" Molecules 19, no. 8: 12011-12030. https://doi.org/10.3390/molecules190812011