Epigalloccatechin-3-gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells via Suppression of MMP-9 and VEGF Activation
Abstract
:1. Introduction

2. Results and Discussion
2.1. EGCG Has Less Cytotoxicity in Retinal Pigment Epithelial Cells and Retinal Microvascular Endothelial Cells

2.2. EGCG Inhibits TPA-Induced MMP-9 Protein Production and mRNA Expression in ARPE-19 Cells

2.3. Inhibition of H2O2-Induced Cell Death and ROS Production by EGCG in ARPE-19 Cells
2.4. EGCG Suppresses Expression of MMP-9, VEGF, and VEGFR-2 on H2O2-Induced Oxidative Stress in ARPE-19 Cells


2.5. EGCG Inhibits VEGF-Induced Proliferation and Vascular Permeability in HRMECs
2.6. Inhibition of VEGF-Induced Tube Formation by EGCG in HRMECs


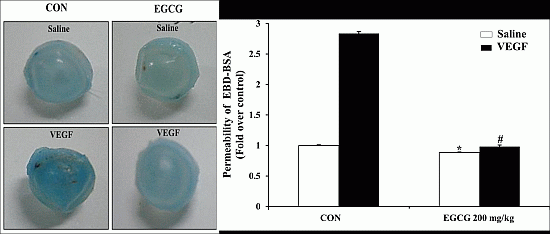
2.7. EGCG Inhibits VEGF-Induced Vascular Permeability in Vivo

2.8. EGCG Inhibits VEGF-Induced Vascular Leakage by Blood-Retinal Barrier Breakdown in Vivo

2.9. EGCG Inhibits Corneal Neovascularization via the Suppression of MMP-9 Activity in an Alkali Burn-Induced Model of Corneal Angiogenesis in Vivo

3. Experimental
3.1. Animals
3.2. Cell Cultures
3.3. Cell Viability
3.4. Gelatin Zymography on ARPE-19 Cells
3.5. RNA Isolation and Quantitative Real-Time PCR
| Gene | Primer | Sequence |
|---|---|---|
| MMP-9 | F | 5'-GAG TGT AAC CAT AGC GGT ACA GG-3' |
| R | 5'-TCT TCC CTG GAG ACC TGA GA-3' | |
| VEGFR-1 | F | 5'-CCA CTC CCT TGA ACA CGA G-3' |
| R | 5'-GTC GCC TTA CGG AAG CTC-3' | |
| VEGFR-2 | F | 5'-GAA CAT TTG GGA AAT CTC TTG C-3' |
| R | 5'-CGG AAG AAC AAT GTA GTC TTT G-3' | |
| GAPDH | F | 5'-CCC CGG TTT CTA TAA ATT GAG C-3' |
| R | 5'-CAC CTT CCC CAT GGT GTC T-3' |
3.6. Detection of MMP-9 Expression Levels on TPA-Induced ARPE-19 Cells
3.7. Cell Viability on H2O2-Induced ARPE-19 Cells
3.8. Intracellular Reactive Oxygen Species (ROS) Assay on ARPE-19 Cells
3.9. Detection of MMP-9, VEGF, and VEGFR-2 Expression Levels on H2O2-Induced ARPE-19 Cells
3.10. VEGF-Induced Proliferation Assay
3.11. VEGF-Induced Tube Formation Assay
3.12. In Vitro Vascular Permeability on HRMECs
3.13. Miles Assay for in Vivo Permeability
3.14. Retinal Vascular Permeability (RVP) Assay
3.15. Corneal Neovascularization (CNV) Assay & Immunohistochemistry (IHC)
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, R.; Fackler, I.; Linsenmaier, U.; Mayer, K.; Licht, T.; Kretzler, M. Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex. Am. J. Kidney Dis. 2006, 48, e27–e29. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, A.; Li, S.; Luo, Y.; Huang, J.; Yu, H.; Ma, W.; Pan, J.; Zhong, Q.; Yang, J.; et al. Kh906, a recombinant human vegf receptor fusion protein, is a new effective topical treatment for corneal neovascularization. Mol. Vis. 2011, 17, 797–803. [Google Scholar] [PubMed]
- Tamary, H.; Offret, H.; Dgany, O.; Foliguet, B.; Wickramasinghe, S.N.; Krasnov, T.; Rumilly, F.; Goujard, C.; Feneant-Thibault, M.; Cynober, T.; et al. Congenital dyserythropoietic anaemia, type I, in a caucasian patient with retinal angioid streaks (homozygous arg1042trp mutation in codanin-1). Eur. J. Haematol. 2008, 80, 271–274. [Google Scholar]
- Gospodarowicz, D.; Cheng, J.; Lirette, M. Bovine brain and pituitary fibroblast growth factors: Comparison of their abilities to support the proliferation of human and bovine vascular endothelial cells. J. Cell Biol. 1983, 97, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. Pdgf-bb modulates endothelial proliferation and angiogenesis in vitro via pdgf beta-receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef] [PubMed]
- McCarty, C.A.; Mukesh, B.N.; Fu, C.L.; Mitchell, P.; Wang, J.J.; Taylor, H.R. Risk factors for age-related maculopathy: The visual impairment project. Arch. Ophthalmol. 2001, 119, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Hjelmeland, L.M.; Handa, J.T. The use of hyperoxia to induce chronic mild oxidative stress in rpe cells in vitro. Mol. Vis. 2001, 7, 63–70. [Google Scholar] [PubMed]
- Abdelsalam, A.; del Priore, L.; Zarbin, M.A. Drusen in age-related macular degeneration: Pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 1999, 44, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. Blood-retinal barriers in health and disease. T. Ophthalmol. Soc. UK 1980, 100, 337–340. [Google Scholar]
- Vinores, S.A.; Derevjanik, N.L.; Vinores, M.A.; Okamoto, N.; Campochiaro, P.A. Sensitivity of different vascular beds in the eye to neovascularization and blood-retinal barrier breakdown in vegf transgenic mice. Adv. Exp. Med. Biol. 2000, 476, 129–138. [Google Scholar] [PubMed]
- Burger, P.C.; Chandler, D.B.; Klintworth, G.K. Experimental corneal neovascularization: Biomicroscopic, angiographic, and morphologic correlation. Cornea 1985, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yaylali, V.; Ohta, T.; Kaufman, S.C.; Maitchouk, D.Y.; Beuerman, R.W. In vivo confocal imaging of corneal neovascularization. Cornea 1998, 17, 646–653. [Google Scholar] [PubMed]
- Qazi, Y.; Maddula, S.; Ambati, B.K. Mediators of ocular angiogenesis. J. Genet. 2009, 88, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Gilbertson-Beadling, S.; Powers, E.A.; Petzold, G.; Poorman, R.; Mitchell, M.A. Interstitial collagenase is required for angiogenesis in vitro. Dev. Biol. 1994, 162, 499–510. [Google Scholar] [CrossRef]
- Schnaper, H.W.; Kleinman, H.K. Regulation of cell function by extracellular matrix. Pediatr. Nephrol. 1993, 7, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McGuire, P.G.; Eriqat, C.; Ober, R.R.; DeJuan, E., Jr.; Williams, G.A.; McLamore, A.; Biswas, J.; Johnson, D.W. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Investig. Ophth. Vis. Sci. 1999, 40, 809–813. [Google Scholar]
- Noda, K.; Ishida, S.; Inoue, M.; Obata, K.; Oguchi, Y.; Okada, Y.; Ikeda, E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest. Ophth. Vis. Sci. 2003, 44, 2163–2170. [Google Scholar] [CrossRef]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix metalloproteinase gelatinase b (mmp-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Drevs, J.; Schneider, V. The use of vascular biomarkers and imaging studies in the early clinical development of anti-tumour agents targeting angiogenesis. J. Int. Med. 2006, 260, 517–529. [Google Scholar]
- Sang, Q.X. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998, 8, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, S3431–S3440. [Google Scholar]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouille, Y.; Seron, K. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis c virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [PubMed]
- Dann, J.M.; Sykes, P.H.; Mason, D.R.; Evans, J.J. Regulation of vascular endothelial growth factor in endometrial tumour cells by resveratrol and egcg. Gynecol. Oncol. 2009, 113, 374–378. [Google Scholar] [CrossRef] [PubMed]
- El-Mowafy, A.M.; Salem, H.A.; Al-Gayyar, M.M.; El-Mesery, M.E.; El-Azab, M.F. Evaluation of renal protective effects of the green-tea (egcg) and red grape resveratrol: Role of oxidative stress and inflammatory cytokines. Nat. Prod. Res. 2011, 25, 850–856. [Google Scholar]
- Oak, M.H.; El Bedoui, J.; Schini-Kerth, V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hasebe-Takenaka, Y.; Ueda, T.; Nakanishi-Ueda, T.; Kosuge, S.; Aburada, M.; Shimada, T.; Ikeya, Y.; Onda, H.; Ogura, H.; et al. Effects of green tea fractions on oxygen-induced retinal neovascularization in the neonatal rat. J. Clin. Biochem. Nutr. 2007, 41, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, T.; Pabst, S.; Stier, S.; Weber, A.A.; Schror, K.; Sachinidis, A.; Vetter, H.; Ko, Y.D. Inhibition of the vascular-endothelial growth factor-induced intracellular signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur. J. Pharmacol. 2004, 483, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Fassina, G.; Vene, R.; Morini, M.; Minghelli, S.; Benelli, R.; Noonan, D.M.; Albini, A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin. Cancer Res. 2004, 10, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Sethi, C.S.; Bailey, T.A.; Luthert, P.J.; Chong, N.H. Matrix metalloproteinase biology applied to vitreoretinal disorders. Br. J. Ophthalmol. 2000, 84, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Steen, B.; Sejersen, S.; Berglin, L.; Seregard, S.; Kvanta, A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2194–2200. [Google Scholar]
- Chao, C.Y.; Lii, C.K.; Hsu, Y.T.; Lu, C.Y.; Liu, K.L.; Li, C.C.; Chen, H.W. Induction of heme oxygenase-1 and inhibition of tpa-induced matrix metalloproteinase-9 expression by andrographolide in mcf-7 human breast cancer cells. Carcinogenesis 2013, 34, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Moulik, S.; Dutta, A.; Choudhury, P.R.; Banerji, A.; Das, S.; Roy, M.; Chatterjee, A. Multifunctional effect of epigallocatechin-3-gallate (egcg) in downregulation of gelatinase-a (mmp-2) in human breast cancer cell line mcf-7. Life Sci. 2009, 84, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.B.; Parchment, R.E.; Lewellyn, A.L. Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation 1991, 46, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hollborn, M.; Grosche, A.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol. Vis. 2014, 20, 242–258. [Google Scholar] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.K.; Guo, W.; Liu, L.; Band, M.A.; Paulson, E.K.; Meydani, M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int. J. Cancer 2006, 118, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Mandriota, S.J.; Vassalli, J.D.; Orci, L.; Montesano, R. Angiogenesis-regulating cytokines: Activities and interactions. Curr. Top. Microbiol. 1996, 213, 31–67. [Google Scholar]
- Ito, Y.; Oike, Y.; Yasunaga, K.; Hamada, K.; Miyata, K.; Matsumoto, S.; Sugano, S.; Tanihara, H.; Masuho, Y.; Suda, T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003, 63, 6651–6657. [Google Scholar] [PubMed]
- Xu, Q.; Qaum, T.; Adamis, A.P. Sensitive blood-retinal barrier breakdown quantitation using evans blue. Investig. Ophthalmol. Vis. Sci. 2001, 42, 789–794. [Google Scholar]
- Carter, R.T.; Kambampati, R.; Murphy, C.J.; Bentley, E. Expression of matrix metalloproteinase 2 and 9 in experimentally wounded canine corneas and spontaneous chronic corneal epithelial defects. Cornea 2007, 26, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.; Chen, J.K.; Kim, W.S.; Hao, Y.X.; Wu, H.C.; Tsai, R.J.; Hwang, D.G.; Zhang, F. Expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinase 1 and 2 in inflammation-induced corneal neovascularization. Ophthalmic Res. 2001, 33, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of mmp-2, mmp-9 and vegf are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006, 26, 3579–3583. [Google Scholar] [PubMed]
- Sample Availability: Samples of EGCG (Teavigo) are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, H.S.; Jun, J.-H.; Jung, E.-H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells via Suppression of MMP-9 and VEGF Activation. Molecules 2014, 19, 12150-12172. https://doi.org/10.3390/molecules190812150
Lee HS, Jun J-H, Jung E-H, Koo BA, Kim YS. Epigalloccatechin-3-gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells via Suppression of MMP-9 and VEGF Activation. Molecules. 2014; 19(8):12150-12172. https://doi.org/10.3390/molecules190812150
Chicago/Turabian StyleLee, Hak Sung, Jae-Hyun Jun, Eun-Ha Jung, Bon Am Koo, and Yeong Shik Kim. 2014. "Epigalloccatechin-3-gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells via Suppression of MMP-9 and VEGF Activation" Molecules 19, no. 8: 12150-12172. https://doi.org/10.3390/molecules190812150