Chitosan Combined with Molecular Beacon for Mir-155 Detection and Imaging in Lung Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis Optimum CS-MB Nanoparticals Complexes

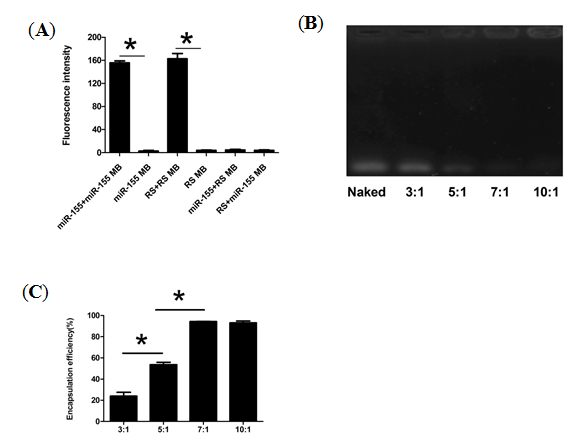
2.2. Physicochemical Characteristics of CS-MB Nanoparticle Complexes

2.3. Fluorescence Imaging and Detecting in Viable Cells

2.4. Flow Cytometry Analysis and Cytotoxicity Assay

3. Experimental Section
3.1. Hybridization Assay
3.2. DNA Retardation Assay
3.3. Encapsulation of Mir-155MB
3.4. Characterizations of Size, Zeta Potential and Morphology
3.5. DNase I Assay
3.6. Confocal Microscopy
3.7. Flow Cytometry and MTT Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Li, J.; Tong, L.; Zhang, J.; Zhai, A.; Xu, K.; Wei, L.; Chu, M. The prognostic value of miR-21 and miR-155 in non-small-cell lung cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2013, 43, 813–820. [Google Scholar] [CrossRef]
- Kim, B.; Lee, H.J.; Choi, H.Y.; Shin, Y.; Nam, S.; Seo, G.; Son, D.S.; Jo, J.; Kim, J.; Lee, J.; et al. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data. Cancer Res. 2007, 67, 7431–7438. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, K.J.; Lee, M.; Jo, M.H.; Kim, S. Molecular imaging of a cancer-targeting theragnostics probe using a nucleolin aptamer- and microRNA-221 molecular beacon-conjugated nanoparticle. Biomaterials 2012, 33, 207–217. [Google Scholar]
- Li, J.; Tan, S.; Kooger, R.; Zhang, C.; Zhang, Y. MicroRNAs as novel biological targets for detection and regulation. Chem. Soc. Rev. 2014, 43, 506–517. [Google Scholar] [CrossRef]
- Kang, W.J.; Cho, Y.L.; Chae, J.R.; Lee, J.D.; Choi, K.J.; Kim, S. Molecular beacon-based bioimaging of multiple microRNAs during myogenesis. Biomaterials 2011, 32, 1915–1922. [Google Scholar]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1236–1243. [Google Scholar] [CrossRef]
- He, J.; Zhang, F.; Wu, Y.; Zhang, W.; Zhu, X.; He, X.; Zhao, Y.; Zhang, W.; Zhao, Y. Prognostic role of microRNA-155 in various carcinomas: Results from a meta-analysis. Dis. Markers 2013, 34, 379–386. [Google Scholar]
- Dagan, L.N.; Jiang, X.; Bhatt, S.; Cubedo, E.; Rajewsky, K.; Lossos, I.S. MiR-155 regulates HGAL expression and increases lymphoma cell motility. Blood 2012, 119, 513–520. [Google Scholar]
- Han, Z.B.; Chen, H.Y.; Fan, J.W.; Wu, J.Y.; Tang, H.M.; Peng, Z.H. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J. Cancer Res. Clin. Oncol. 2012, 138, 153–161. [Google Scholar]
- Xie, Q.; Chen, X.; Lu, F.; Zhang, T.; Hao, M.; Wang, Y.; Zhao, J.; McCrae, M.A.; Zhuang, H. Aberrant express ion of microRNA 155 may accelerate cell proliferation by targeting sex-determining region Y box 6 in hepatocellular carcinoma. Cancer 2012, 118, 2431–2442. [Google Scholar]
- Zheng, D.; Haddadin, S.; Wang, Y.; Gu, L.Q.; Perry, M.C.; Freter, C.E.; Wang, M.X. Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int. J. Clin. Exp. Pathol. 2011, 4, 575–586. [Google Scholar]
- Kim, W.J.; Kim, S.W. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm. Res. 2009, 26, 657–666. [Google Scholar]
- Yao, Q.; Sun, J.G.; Ma, H.; Zhang, A.M.; Lin, S.; Zhu, C.H.; Zhang, T.; Chen, Z.T. Monitoring microRNAs using a molecular beacon in CD133+/CD338+ human lung adenocarcinoma-initiating A549 cells. Asian Pac. J. Cancer. Prev. 2014, 15, 161–166. [Google Scholar] [CrossRef]
- Geng, Y.; Lin, D.; Shao, L.; Yan, F.; Ju, H. Cellular delivery of quantum dot-bound hybridization probe for detection of intracellular pre-microRNA using chitosan/poly(γ-glutamic acid) complex as a carrier. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Zhang, H.; Meng, X.; Ai, S. Electrochemical determination of microRNA-21 based on graphene, LNA integratedmolecular beacon, AuNPs and biotin multifunctional bio bar codes and enzymaticassay system. Biosens. Bioelectron. 2012, 33, 247–253. [Google Scholar]
- Kam, Y.; Rubinstein, A.; Nissan, A.; Halle, D.; Yavin, E. Detection of endogenous K-ras mRNA in living cells at a single base resolution by aPNA molecular beacon. Mol. Pharm. 2012, 9, 685–693. [Google Scholar]
- Xiang, D.; Zhang, C.; Chen, L.; Ji, X.; He, Z. Tricolour fluorescence detection of sequence-specificDNA with a new molecular beacon and a nucleic acid dye TOTO-3. Analyst 2012, 137, 5898–5905. [Google Scholar]
- Baker, M.B.; Bao, G.; Searles, C.D. The use of molecular beacons to detect and quantify microRNA. Methods Mol. Biol. 2013, 1039, 279–287. [Google Scholar]
- Chen, T.; Wu, C.S.; Jimenez, E.; Zhu, Z.; Dajac, J.G.; You, M.; Han, D.; Zhang, X.; Tan, W. DNA micelle flares for intracellular mRNA imaging and gene therapy. Angew. Chem. Int. Ed. Engl. 2013, 52, 2012–2016. [Google Scholar]
- Butterworth, K.T.; McMahon, S.J.; Taggart, L.E.; Prise, K.M. Radiosensitization by gold nanoparticles: Effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013, 2, 269–279. [Google Scholar]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar]
- Plianwong, S.; Opanasopit, P.; Ngawhirunpat, T.; Rojanarata, T. Chitosan combined with poly-l-arginine as efficient, safe, and serum-insensitive vehicle with RNase protection ability for siRNA delivery. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kim, J.S.; Lee, M.R.; Jeong, H.S.; Kim, J. A study of microRNAs in silico and in vivo: Emerging regulators of embryonic stem cells. FEBS J. 2009, 276, 2140–2149. [Google Scholar]
- Oliveira, A.V.; Silva, A.P.; Bitoque, D.B.; Silva, G.A.; da Costa, R.A.M. Transfection efficiency of chitosan and thiolated chitosan in retinal pigment epithelium cells: A comparative. J. Pharm. Bioallied Sci. 2013, 5, 111–118. [Google Scholar]
- Csaba, N.; Köpping-Höggård, M.; Alonso, M.J. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int. J. Pharm. 2009, 382, 205–214. [Google Scholar]
- Mansouri, S.; Lavigne, P.; Corsi, K.; Benderdour, M.; Beaumont, E.; Fernandes, J.C. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: Strategies to improve transfection efficacy. Eur. J. Pharm. Biopharm. 2004, 57, 1–8. [Google Scholar]
- Borges, O.; Tavares, J.; de Sousa, A.; Borchard, G.; Junginger, H.E.; Cordeiro-da-Silva, A. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur. J. Pharm. Sci. 2007, 32, 278–290. [Google Scholar]
- Raftery, R.; O’Brien, F.J.; Cryan, S.A. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 2013, 18, 5611–5647. [Google Scholar]
- Griveau, A.; Bejaud, J.; Anthiya, S.; Avril, S.; Autret, D.; Garcion, E. Silencing of miR-21 by locked nucleic acid-lipid nanocapsule complexes sensitizehuman glioblastoma cells to radiation-induced cell death. Int. J. Pharm. 2013, 454, 765–774. [Google Scholar]
- Martinez, K.; Estevez, M.C.; Wu, Y.; Phillips, J.A.; Medley, C.D.; Tan, W. Locked nucleic acid based beacons for surface interaction studies and biosensor development. Anal. Chem. 2009, 81, 3448–3454. [Google Scholar]
- Doessing, H.; Vester, B. Locked and unlocked nucleosides in functional nucleic acids. Molecules 2011, 16, 4511–4526. [Google Scholar]
- Yao, Q.; Zhang, A.M.; Ma, H.; Lin, S.; Wang, X.X.; Sun, J.G.; Chen, Z.T. Novel molecular beacons tomonitor microRNAs in non-small-cell lung cancer. Mol. Cell. Probes 2012, 26, 182–187. [Google Scholar]
- Ilieva, M.; Vedova, D.P.; Hansen, O.; Dufva, M. Tracking neuronal marker expression inside living differentiating cells using molecular beacons. Front. Cell. Neurosci. 2013, 19, 266. [Google Scholar]
- Johnson, B.N.; Mutharasan, R. Biosensor-based microRNA detection: Techniques, design, performance and challenges. Analyst 2014, 139, 1576–1588. [Google Scholar]
- Choi, Y.; Kim, H.S.; Woo, J.; Hwang, E.H.; Cho, K.W.; Kim, S.; Moon, W.K. Real-time imaging of the epithelial-mesenchymal transition using microRNA-200a sequence-based molecular beacon-conjugated magnetic nanoparticles. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, H.-Z.; An, J.-H.; Yao, Q.; Han, J.; Li, X.-T.; Jiang, F.-L.; Chen, G.-P.; Peng, L.-N.; Li, Y.-S.; Sun, J.-G.; et al. Chitosan Combined with Molecular Beacon for Mir-155 Detection and Imaging in Lung Cancer. Molecules 2014, 19, 14710-14722. https://doi.org/10.3390/molecules190914710
Zhu H-Z, An J-H, Yao Q, Han J, Li X-T, Jiang F-L, Chen G-P, Peng L-N, Li Y-S, Sun J-G, et al. Chitosan Combined with Molecular Beacon for Mir-155 Detection and Imaging in Lung Cancer. Molecules. 2014; 19(9):14710-14722. https://doi.org/10.3390/molecules190914710
Chicago/Turabian StyleZhu, Hai-Zhen, Jiang-Hong An, Quan Yao, Jing Han, Xue-Tao Li, Fei-Long Jiang, Guang-Peng Chen, Li-Na Peng, Yong-Sheng Li, Jian-Guo Sun, and et al. 2014. "Chitosan Combined with Molecular Beacon for Mir-155 Detection and Imaging in Lung Cancer" Molecules 19, no. 9: 14710-14722. https://doi.org/10.3390/molecules190914710