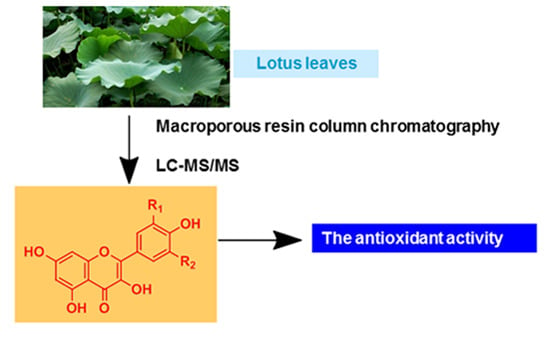
Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Flavonoids by HPLC-MS/MS


| Peak No. | Rt (min) a | NI-MS | MS/MS | Identification |
|---|---|---|---|---|
| 1 | 25.9 | 479 | 316 | Myricetin 3-O-hexose |
| 2 | 27.4 | 595 | 300 | Quercetin 3-O-arabinopyranosyl-(1→2)-galactopyranoside |
| 3 | 33.0 | 609 | 300 | Quercetin 3-O-rhamnopyranosyl-(1→2)-glucopyranoside |
| 4 | 34.1 | 463 | 300 | Quercetin 3-O-galactoside (hyperoside) |
| 5 | 35.5 | 463 | 300 | Quercetin 3-O-glucoside (isoquercitrin) |
| 6 | 37.2 | 477 | 301 | Quercetin 3-O-glucuronide |
| 7 | 38.7 | 433 | 300 | Quercetin 3-O-arabinoside |
| 8 | 40.6 | 447 | 284 | Kaempferol 3-O-galactoside |
| 9 | 43.7 | 447 | 284 | Kaempferol 3-O-glucoside (astragalin) |
| 10 | 45.7 | 461 | 285 | Kaempferol 3-O-glucuronide |
| 11 | 47.5 | 461 | 446; 298; 283 | Diosmetin 7-O-hexose |
| 12 | 52.6 | 609 | 314; 299 | Isorhamnetin 3-O-arabinopyranosyl-(1→2)-glucopyranoside |
| 13 | 56.7 | 477 | 314 | Isorhamnetin 3-O-hexose |
| 14 | 57.9 | 491 | 315 | Isorhamnetin 3-O-glucuronide |

2.2. Antioxidant Activity of Flavonoids from Lotus Leaves
| Sample | Total Flavonoid (mg IE/g) | DPPH | ABTS | FRAP (mmol Fe2+/100 g) | ||
|---|---|---|---|---|---|---|
| (μmol TE/g) | IC50 value b (mg/mL) | (μmol TE/g) | IC50 value b (mg/mL) | |||
| Fraction II | 690.5 ± 35.8 | 4695.3 ± 144.3 | 0.101 ± 0.007 | 5012.3 ± 133.8 | 0.138 ± 0.007 | 500.5 ± 62.8 |
| BHT | nt | 3612.3 ± 170.9 | 0.121 ± 0.004 | 4567.0 ± 155.6 | 0.143 ± 0.004 | nt |
| Trolox | nt | nt | 0.112 ± 0.005 | nt | 0.119 ± 0.005 | 642.1 ± 55.7 |
3. Experimental Section
3.1. Chemicals and Materials
3.2. Extraction and Fractionation of Crude Lotus Leaves Extracts
3.3. Determinations of Total Flavonoids Content (TFC)
3.4. HPLC Analysis of Flavonoids
3.5. Identification of Flavonoids
3.6. Determination of Antioxidant Activity of Flavonoids
3.6.1. DPPH Free Radical Scavenging Activity
3.6.2. ABTS Free Radical Scavenging Activity
3.6.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)—Phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Kredy, H.M.; Huang, D.H.; Xie, B.J.; He, H.; Yang, E.N.; Tian, B.Q.; Xiao, D. Flavonols of lotus (Nelumbo nucifera, gaertn.) seed epicarp and their antioxidant potential. Eur. Food Res. Technol. 2010, 231, 387–394. [Google Scholar] [CrossRef]
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; pp. 258–259. [Google Scholar]
- Wu, M.J.; Wang, L.; Weng, C.Y.; Yen, J.H. Antioxidant activity of methanol extract of the lotus leaf (Nelumbo nucifera gertn.). Am. J. Chin. Med. 2003, 31, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, E.; Miyazaki, H.; Shindo, K.; Watanabe, H.; Yoshida, A.; Yajima, H. Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. Planta Med. 2007, 73, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ban, X.Q.; He, J.S.; Tong, J.; Tian, J.; Wang, Y.W. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Luo, X.B.; Chen, B.; Liu, J.J.; Yao, S.Z. Simultaneous analysis of N-nornuciferine, O-nornuciferine, nuciferine, and roemerine in leaves of Nelumbo nucifera gaertn by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. Anal. Chim. Acta 2005, 538, 129–133. [Google Scholar] [CrossRef]
- Elegami, A.A.; Bates, C.; Gray, A.I.; Mackay, S.P.; Skellern, G.G.; Waigh, R.D. Two very unusual macrocyclic flavonoids from the water lily Nymphaea lotus. Phytochemistry 2003, 63, 727–731. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, U. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Araiza, C.; Alvarez-Mejia, A.L.; Sanchez-Torres, S.; Farfan-Garcia, E.; Mondragon-Lozano, R.; Pinto-Almazan, R.; Salgado-Ceballos, H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013, 47, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta. 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Dikshit, M.; Flora, G. Role of free radicals and antioxidants in health and disease. Cell. Mol. Biol. 2007, 53, 1–3. [Google Scholar] [PubMed]
- Wan, P.F.; Sheng, Z.L.; Han, Q.; Zhao, Y.L.; Cheng, G.D.; Li, Y.H. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B 2014, 945, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.P.; Lu, Y.H.; Wei, D.Z. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, B.H.; Fang, J.B.; Liu, Y.L.; Zhang, H.H.; Fang, L.C.; Guan, L.; Li, S.H. Analysis of flavonoids from lotus (Nelumbo nucifera) leaves using high performance liquid chromatography/photodiode array detector tandem electrospray ionization mass spectrometry and an extraction method optimized by orthogonal design. J. Chromatogr. A 2012, 1227, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.G.; Deng, Z.Y.; Fan, Y.W.; Peng, Y.; Li, J.; Xiong, D.M.; Liu, R. Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (lotus) by high-speed counter-current chromatography. J. Chromatogr. B 2009, 877, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wang, K.; Tao, Y.; Chen, L.; Qiu, F. Purification of baicalin and wogonoside from Scutellaria baicalensis extracts by macroporous resin adsorption chromatography. J. Chromatogr. B 2012, 908, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chase, H.A. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat. Prod. Rep. 2010, 27, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.S.; Cai, Z.W. Chemical investigation on Sijunzi decoction and its two major herbs Panax ginseng and Glycyrrhiza uralensis by LC/MS/MS. J. Pharmaceut. Biomed. 2006, 41, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, L.C.; Xi, H.F.; Guan, L.; Fang, J.B.; Liu, Y.L.; Wu, B.H.; Li, S.H. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal. Chim. Acta 2012, 724, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.R.; Choi, J.S.; Na, D.H. Simultaneous determination of quercetin and its glycosides from the leaves of Nelumbo nucifera by reversed-phase high-performance liquid chromatography. Arch. Pharm. Res. 2009, 32, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yan, C.Y.; Li, L.; Liu, Z.Q.; Liu, S.Y. Studies on the flavones using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2004, 1047, 213–220. [Google Scholar] [CrossRef]
- Abad-Garcia, B.; Berrueta, L.A.; Garmon-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xing, J.P.; Liu, S.; Song, F.R.; Cai, Z.W.; Pi, Z.F.; Liu, Z.Q.; Liu, S.Y. Screening and determination for potential a-glucosidase inhibitors from leaves of acanthopanax senticosus harms by using UF-LC/MS and ESI-MSn. Phytochem. Anal. 2012, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Z.; Wei, X.L.; Gao, F.F.; Wang, L.S.; Zhang, H.J.; Xu, Y.J.; Li, C.H.; Ge, Y.X.; Zhang, J.J.; Zhang, J. Simultaneous analysis of anthocyanins and flavonols in petals of lotus (Nelumbo) cultivars by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry. J. Chromatogr. A 2009, 1216, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.H.; Jiang, Z.T.; Liu, T.; Li, R. Flavonoids in juglans regia l. Leaves and evaluation of in vitro antioxidant activity via intracellular and chemical methods. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Bio. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- RiceEvans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Bio. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Zou, Y.P.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant activity and phenolic compositions of Lentil (Lens culinaris var. Morton) extract and its fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Brandwilliams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds (quercetin 3-O-galactoside (hyperoside), quercetin 3-O-glucoside (isoquercitrin), kaempferol 3-O-glucoside (astragalin)) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.-Z.; Wu, W.; Jiao, L.-L.; Yang, P.-F.; Guo, M.-Q. Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays. Molecules 2015, 20, 10553-10565. https://doi.org/10.3390/molecules200610553
Zhu M-Z, Wu W, Jiao L-L, Yang P-F, Guo M-Q. Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays. Molecules. 2015; 20(6):10553-10565. https://doi.org/10.3390/molecules200610553
Chicago/Turabian StyleZhu, Ming-Zhi, Wei Wu, Li-Li Jiao, Ping-Fang Yang, and Ming-Quan Guo. 2015. "Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays" Molecules 20, no. 6: 10553-10565. https://doi.org/10.3390/molecules200610553