Some Phthalocyanine and Naphthalocyanine Derivatives as Corrosion Inhibitors for Aluminium in Acidic Medium: Experimental, Quantum Chemical Calculations, QSAR Studies and Synergistic Effect of Iodide Ions
Abstract
:1. Introduction


2. Results and Discussion
2.1. Gravimetric Measurements
2.1.1. Effect of Inhibitor Concentration and Temperature



2.1.2. Thermodynamic and Activation Parameters
| Without KI | With KI | ||||||
|---|---|---|---|---|---|---|---|
| Inhibitor | Conc. (ppm) | Ea (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (JK−1·mol−1) | Ea (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J·K−1·mol−1) |
| Blank | - | 10.55 | 167.00 | −275.79 | 10.55 | 167.00 | −275.79 |
| Pc1 | 25 | 8.68 | 137.38 | −283.30 | 8.74 | 138.38 | −277.32 |
| 50 | 7.49 | 118.47 | −287.40 | 7.70 | 121.86 | −278.44 | |
| 75 | 7.70 | 121.79 | −286.77 | 7.21 | 114.17 | −275.74 | |
| 100 | 9.09 | 143.91 | −284.52 | 8.77 | 138.76 | −276.47 | |
| Pc2 | 25 | 11.41 | 180.62 | −274.39 | 11.72 | 185.54 | −273.75 |
| 50 | 11.82 | 186.99 | −273.21 | 12.38 | 195.97 | −271.76 | |
| 75 | 11.68 | 184.80 | −273.76 | 12.14 | 192.07 | −272.68 | |
| 100 | 11.58 | 183.24 | −274.13 | 12.08 | 191.25 | −272.45 | |
| Pc3 | 25 | 11.50 | 181.92 | −274.10 | 11.88 | 187.96 | −273.22 |
| 50 | 11.52 | 182.29 | −274.08 | 11.71 | 185.26 | −273.74 | |
| 75 | 11.73 | 185.60 | −273.60 | 12.05 | 190.72 | −272.89 | |
| 100 | 11.10 | 175.71 | −275.68 | 11.65 | 184.43 | −273.33 | |
| Pc4 | 25 | 11.70 | 185.22 | −273.51 | 12.64 | 200.11 | −270.91 |
| 50 | 12.10 | 191.51 | −272.39 | 12.33 | 195.18 | −271.94 | |
| 75 | 11.94 | 188.97 | −273.02 | 12.18 | 192.70 | −272.54 | |
| 100 | 11.47 | 181.56 | −274.66 | 12.26 | 194.04 | −272.50 | |
| nPc1 | 25 | 10.39 | 164.37 | −277.80 | 10.63 | 168.28 | −277.32 |
| 50 | 10.18 | 161.12 | −278.56 | 10.31 | 163.18 | −278.44 | |
| 75 | 10.74 | 169.99 | −276.90 | 11.21 | 177.47 | −275.74 | |
| 100 | 10.57 | 167.30 | −277.66 | 11.05 | 174.94 | −276.47 | |
| nPc2 | 25 | 11.11 | 175.76 | −275.36 | 11.64 | 184.28 | −273.90 |
| 50 | 11.97 | 189.39 | −272.82 | 12.31 | 194.75 | −272.02 | |
| 75 | 11.40 | 180.42 | −274.72 | 12.03 | 190.42 | −273.09 | |
| 100 | 10.34 | 163.60 | −278.29 | 10.92 | 172.76 | −276.74 | |
| nPc3 | 25 | 7.97 | 126.09 | −285.33 | 8.01 | 126.67 | −285.49 |
| 50 | 8.42 | 133.30 | −284.17 | 8.66 | 133.30 | −283.52 | |
| 75 | 8.31 | 131.49 | −282.54 | 8.39 | 131.49 | −282.27 | |
| 100 | 8.89 | 140.76 | −282.86 | 9.18 | 140.76 | −282.28 | |
2.2. Adsorption Isotherms

2.3. Potentiodynamic Polarization Measurements
| Without KI | With KI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inhibitor | T/K | Kads (103 × mol−1) | −ΔG°ads (kJ∙mol−1) | ΔH° (kJ∙mol−1) | ΔS° (kJ∙mol−1) | Kads (103 × mol−1) | −ΔG°ads (kJ∙mol−1) | ΔH° (kJ∙mol−1) | ΔS° (kJ·mol−1) |
| Pc1 | 303 | 22.99 | −18.02 | 18.06 | 0.12 | 69.69 | −20.81 | 21.56 | 2.46 |
| 313 | 76.34 | −21.04 | 21.08 | 84.75 | −21.31 | 22.08 | |||
| 323 | 48.78 | −19.91 | 19.95 | 85.47 | −21.33 | 22.12 | |||
| 333 | 55.25 | −20.23 | 20.27 | 136.99 | −22.52 | 23.34 | |||
| 343 | 312.50 | −24.59 | 24.64 | 180.18 | −23.21 | 24.05 | |||
| Pc2 | 303 | 15.65 | −17.05 | 17.08 | 0.09 | 42.28 | −19.55 | 21.07 | 5.00 |
| 313 | 34.25 | −19.02 | 19.05 | 48.19 | −19.88 | 21.45 | |||
| 323 | 90.09 | −21.46 | 21.49 | 61.54 | −20.50 | 22.11 | |||
| 333 | 99.01 | −21.70 | 21.73 | 76.28 | −21.04 | 22.70 | |||
| 343 | 51.68 | −20.06 | 20.09 | 86.96 | −21.37 | 23.08 | |||
| Pc3 | 303 | 17.41 | −17.32 | 17.34 | 0.09 | 17.41 | −20.27 | 23.08 | 9.27 |
| 313 | 24.54 | −18.18 | 18.21 | 24.54 | −20.45 | 23.35 | |||
| 323 | 40.57 | −19.45 | 19.48 | 40.57 | −20.68 | 23.67 | |||
| 333 | 86.96 | −21.37 | 21.40 | 86.96 | −21.08 | 24.17 | |||
| 343 | 55.71 | −20.25 | 20.28 | 55.71 | −21.75 | 24.93 | |||
| Pc4 | 303 | 9.38 | −15.76 | 15.79 | 0.11 | 54.95 | −20.21 | 25.33 | 16.88 |
| 313 | 14.42 | −16.84 | 16.88 | 58.14 | −20.36 | 25.64 | |||
| 323 | 35.03 | −19.08 | 19.11 | 56.66 | −20.29 | 25.74 | |||
| 333 | 40.98 | −19.48 | 19.51 | 61.35 | −20.49 | 26.11 | |||
| 343 | 46.84 | −19.81 | 19.85 | 66.45 | −20.69 | 26.48 | |||
| nPc1 | 303 | 54.94 | −20.21 | 20.22 | 0.01 | 65.15 | −20.64 | 19.45 | −3.95 |
| 313 | 51.68 | −20.06 | 20.06 | 67.11 | −20.72 | 19.48 | |||
| 323 | 64.72 | −20.63 | 20.63 | 81.30 | −21.20 | 19.93 | |||
| 333 | 76.63 | −21.05 | 21.06 | 101.01 | −21.75 | 20.43 | |||
| 343 | 59.00 | −20.39 | 20.40 | 256.41 | −24.10 | 22.74 | |||
| nPc2 | 303 | 17.41 | −17.32 | 17.35 | 0.10 | 93.90 | −21.56 | 28.91 | 24.25 |
| 313 | 19.36 | −17.59 | 17.62 | 134.23 | −22.46 | 30.05 | |||
| 323 | 53.33 | −20.14 | 20.17 | 82.99 | −21.25 | 29.08 | |||
| 333 | 70.92 | −20.86 | 20.89 | 92.59 | −21.53 | 29.60 | |||
| 343 | 66.89 | −20.71 | 20.74 | 96.62 | −21.64 | 29.95 | |||
| nPc3 | 303 | 47.28 | −19.84 | 19.86 | 0.09 | 108.70 | −21.93 | 28.72 | 22.41 |
| 313 | 51.95 | −20.07 | 20.10 | 128.21 | −22.35 | 29.36 | |||
| 323 | 20.41 | −17.72 | 17.75 | 99.50 | −21.71 | 28.95 | |||
| 333 | 116.96 | −22.12 | 22.15 | 116.96 | −22.12 | 29.58 | |||
| 343 | 173.91 | −23.12 | 23.15 | 111.11 | −21.99 | 29.67 | |||
| Without KI | With KI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor | Conc. (ppm) | −Ecorr (mV) | icorr (× 10−3) (µA) | bc (mV/dec) | ba (mV/dec) | % IEP | −Ecorr (mV) | icorr (× 10−3) (µA) | bc (mV/dec) | ba (mV/dec) | % IEP |
| Blank | - | 742 | 1792 | 29 | 165 | - | 742 | 1792 | 29 | 165 | - |
| Pc1 | 25 | 706 | 420 | 23 | 121 | 76.5 | 698 | 273 | 13 | 111 | 84.5 |
| 50 | 707 | 334 | 20 | 167 | 81.4 | 695 | 393 | 14 | 128 | 84.8 | |
| 100 | 705 | 289 | 15 | 137 | 83.9 | 680 | 173 | 14 | 71 | 90.3 | |
| Pc2 | 25 | 707 | 532 | 34 | 154 | 70.3 | 705 | 409 | 11 | 110 | 77.2 |
| 50 | 706 | 398 | 14 | 91 | 77.8 | 709 | 226 | 9 | 63 | 87.4 | |
| 100 | 695 | 269 | 18 | 100 | 85.0 | 708 | 405 | 17 | 124 | 77.4 | |
| Pc3 | 25 | 704 | 290 | 13 | 160 | 83.8 | 698 | 301 | 13 | 120 | 83.2 |
| 50 | 710 | 332 | 12 | 139 | 81.5 | 700 | 421 | 2 | 137 | 76.5 | |
| 100 | 711 | 451 | 20 | 136 | 74.8 | 695 | 235 | 10 | 128 | 86.9 | |
| Pc4 | 25 | 437 | 832 | 78 | 79 | 83.0 | 704 | 398 | 17 | 115 | 77.8 |
| 50 | 735 | 1209 | 24 | 146 | 76.9 | 705 | 373 | 13 | 131 | 79.2 | |
| 100 | 731 | 1031 | 21 | 147 | 74.2 | 684 | 152 | 24 | 117 | 91.5 | |
| nPc1 | 25 | 709 | 101 | 51 | 200 | 70.0 | 700 | 221 | 17 | 119 | 87.7 |
| 50 | 705 | 294 | 11 | 138 | 86.1 | 699 | 324 | 33 | 142 | 81.9 | |
| 100 | 704 | 368 | 18 | 136 | 71.2 | 698 | 253 | 17 | 120 | 85.9 | |
| nPc2 | 25 | 704 | 280 | 10 | 104 | 84.3 | 671 | 17 | 11 | 46 | 99.1 |
| 50 | 709 | 266 | 12 | 58 | 85.1 | 705 | 305 | 12 | 85 | 82.1 | |
| 100 | 707 | 158 | 29 | 147 | 91.2 | 707 | 506 | 13 | 125 | 71.0 | |
| nPc3 | 25 | 704 | 496 | 15 | 165 | 72.1 | 456 | 780 | 38 | 85 | 56.5 |
| 50 | 701 | 613 | 29 | 105 | 65.3 | 685 | 116 | 19 | 124 | 93.5 | |
| 100 | 708 | 681 | 13 | 105 | 96.2 | 676 | 8 | 16 | 42 | 99.6 | |
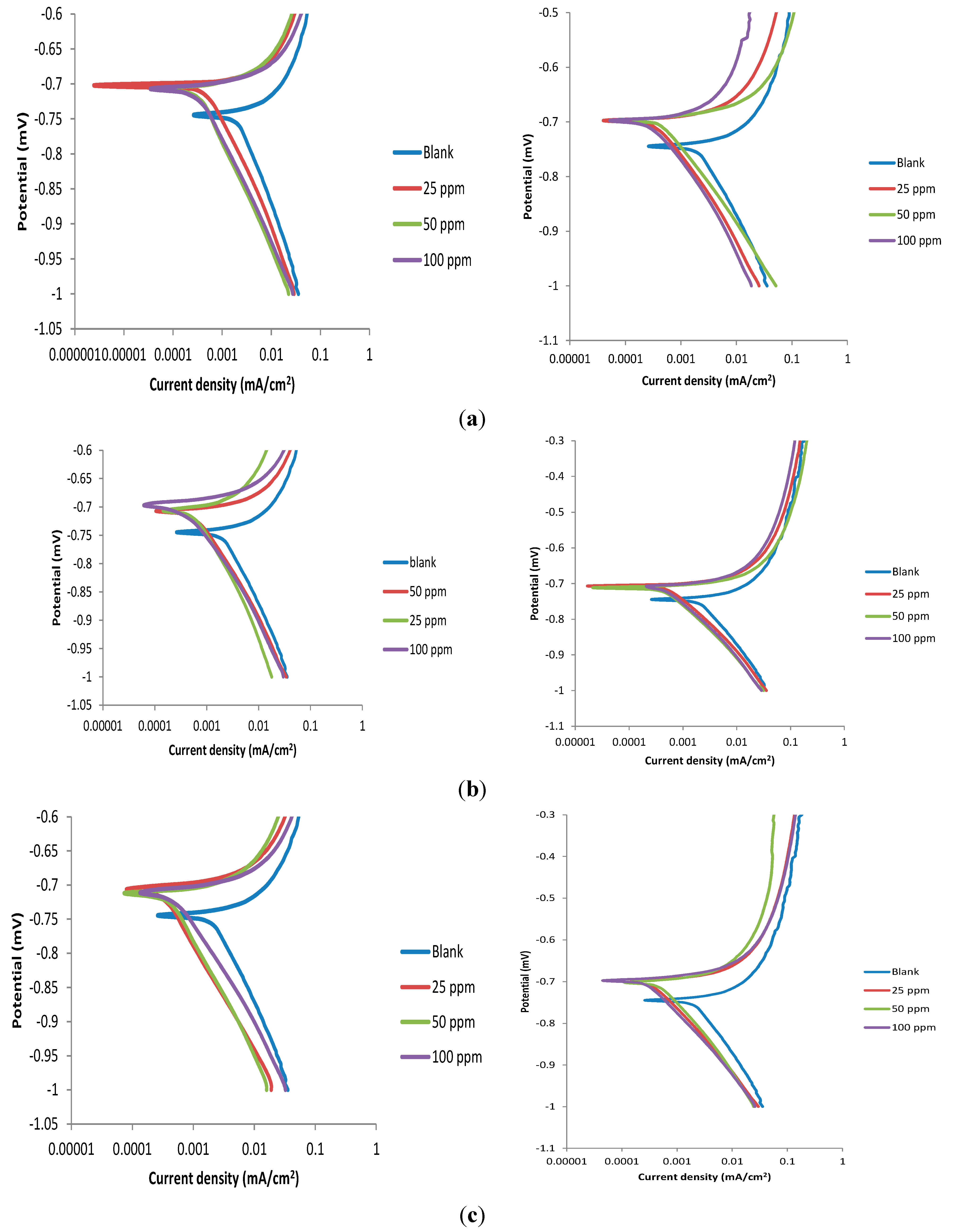


2.4. Synergism Consideration
| Synergistic Parameter (SI) | |||
|---|---|---|---|
| Inhibitor | 25 ppm | 50 ppm | 100 ppm |
| Pc1 | 1.42 (1.53) | 1.35 (1.58) | 1.28 (1.51) |
| Pc2 | 1.49 (1.59) | 1.46 (1.49) | 1.37 (1.78) |
| Pc3 | 1.44 (1.64) | 1.45 (1.75) | 1.37 (1.46) |
| Pc4 | 1.53 (1.74) | 1.36 (1.64) | 1.24 (1.38) |
| nPc1 | 1.23 (1.40) | 1.21 (1.70) | 1.25 (1.44) |
| nPc2 | 1.43 (1.38) | 1.45 (1.68) | 1.32 (2.03) |
| nPc3 | 1.24 (2.22) | 1.21 (1.26) | 1.25 (1.49) |
2.5. Quantum Chemical Calculations




| Reactivity Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | EHOMO | ELUMO | ∆E | η | σ | ∆N | ω | μ | %IE a |
| Pcs | |||||||||
| Pc1 | −4.11 | −2.20 | 1.91 | 0.955 | 1.05 | −2.01 | 5.21 | 1.09 | 67.6 (93.3) |
| Pc2 | −4.44 | −2.27 | 2.17 | 1.085 | 0.92 | −1.68 | 5.19 | 0.03 | 52.3 (76.5) |
| Pc3 | −4.80 | −2.67 | 2.13 | 1.065 | 0.94 | −1.53 | 6.55 | 0.16 | 54.3 (77.9) |
| Pc4 | −4.98 | −2.83 | 2.15 | 1.075 | 0.93 | −1.44 | 7.09 | 0.01 | 46.2 (79.5) |
| nPcs | |||||||||
| nPc1 | −4.25 | −2.58 | 1.67 | 0.835 | 1.20 | −2.15 | 6.98 | 0.09 | 56.8 (87.3) |
| nPc2 | −4.43 | −2.46 | 1.97 | 0.985 | 1.02 | −1.80 | 6.02 | 0.00 | 51.7 (78.6) |
| nPc3 | −4.55 | −2.76 | 1.79 | 0.895 | 1.12 | −1.87 | 7.46 | 0.00 | 61.3 (82.5) |
2.6. Quantitative Structure Activity Relationship (QSAR)

3. Experimental Section
3.1. Materials and Aggressive Solutions
3.2. Gravimetric Method
3.3. Electrochemical Measurements
3.4. Quantum Chemical Studies and Quantitative Structure Activity Relationship (QSAR)
4. Conclusions
- (1)
- All the studied Pcs behave as good corrosion inhibitors for Al in 1 M HCl solution with Pc1 being the best inhibitor among the Pcs and nPc3 being the best among the nPcs.
- (2)
- The addition of potassium iodide (KI) to the Pc and nPc solutions increases the inhibition efficiency.
- (3)
- The adsorption of the studied compounds on Al surface obeys the Langmuir adsorption isotherm.
- (4)
- The thermodynamic and kinetic parameters revealed that the adsorption of the studied compounds on Al surface is spontaneous and involves both physisorption and chemisorption mechanisms.
- (5)
- The quantum chemical parameters showed that the studied Pcs and nPcs have the ability to donate/accept electrons to/from appropriate p and/or d orbitals of metal atoms and support their good corrosion inhibition potentials.
- (6)
- The experimental results revealed the possibility of aggregative interactions between the inhibitor molecules and the results further indicated that these interactions are affected by the peripheral groups on the compounds.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Nie, X.; Wang, L.; Northwood, D.O. Corrosion protection properties of anodic oxide coatings on an Al-Si alloy. Surf. Coat. Technol. 2005, 200, 1994–2000. [Google Scholar] [CrossRef]
- Khaled, K.F.; Amin, M.A. Electrochemical and molecular dynamics simulation studies on the corrosion inhibition of aluminum in molar hydrochloric acid using some imidazole derivatives. J. Appl. Electrochem. 2009, 39, 2553–2568. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Olivares-Xometl, O.; Guzmán-Lucero, D.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Lijanova, I.V.; Arce-Estrada, E. The inhibition of aluminium corrosion in sulfuric acid by poly(1-vinyl-3-alkyl-imidazolium hexafluorophosphate). Materials 2014, 7, 5711–5734. [Google Scholar] [CrossRef]
- Muniandy, M.T.; Rahim, A.A.; Osman, H.; Shah, A.M.; Yahya, S.; Raja, P.B. Investigation of some schiff bases as corrosion inhibitors for aluminium alloy in 0.5 M hydrochloric acid solutions. Surf. Rev. Lett. 2011, 18, 127–133. [Google Scholar] [CrossRef]
- Cabot, P.L.; Centellas, F.A.; Garrido, J.A.; Pérez, E.; Vidal, H. Electrochemical study of aluminium corrosion in acid chloride solutions. Electrochim. Acta 1991, 36, 179–187. [Google Scholar] [CrossRef]
- Brett, C.M.A. On the electrochemical behaviour of aluminium in acidic chloride solution. Corros. Sci. 1992, 33, 203–210. [Google Scholar] [CrossRef]
- Sastri, V.S. Corrosion Inhibitors: Principles and Applications; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Bregman, J.I. Corrosion Inhibitors; Collier-MacMillan Co.: London, UK, 1963. [Google Scholar]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Gajek, A.; Zakroczymski, T.; Romanchuk, V.; Topilnytsky, P. Protective properties and spectral analysis of nitrogen- and oxygen-containing corrosion inhibitors for oil equipment. Chem. Chem. Technol. 2012, 6, 209–217. [Google Scholar]
- Guzman-Lucero, D.; Olivares-Xometl, O.; Martinez-Palou, R.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Garibay-Febles, V. Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 2011, 50, 7129–7140. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313–1321. [Google Scholar] [CrossRef]
- Mazhar, M.A.; Badawy, W.A.; Abou Romia, R.M. Impedance studies of corrosion resistance of aluminium in chloride media. Surf. Coat. Technol. 1986, 29, 335–345. [Google Scholar] [CrossRef]
- Tomcsányi, L.; Varga, K.; Bartik, I.; Horányi, H.; Maleczki, E. Electrochemical study of the pitting corrosion of aluminium and its alloys—II. Study of the interaction of chloride ions with a passive film on aluminium and initiation of pitting corrosion. Electrochim. Acta 1989, 34, 855–859. [Google Scholar] [CrossRef]
- Rio, Y.; Rodríguez-Morgade, M.S.; Torres, T. Modulating the electronic properties of porphyrinoids: A voyage from the violet to the infrared regions of the electromagnetic spectrum. Org. Biomol. Chem. 2008, 6, 1877–1894. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, M.V.; de la Torre, G.; Torres, T. Lighting porphyrins and phthalocyanines for molecular photovoltaics. Chem. Commun. 2010, 46, 7090–7108. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Trukhina, O.; Ince, M.; Torres, T. Towards artificial photosynthesis: Supramolecular, donor-acceptor, porphyrin and phthalocyanine/carbon nanostructure ensembles. Coord. Chem. Rev. 2012, 256, 2453–2477. [Google Scholar] [CrossRef]
- Wöhrle, D.; Suvorova, O.; Gerdes, R.; Bartels, O.; Lapok, L.; Baziakina, N.; Makarov, S.; Slodek, A. Efficient oxidations and photooxidations with molecular oxygen using metal phthalocyanines as catalysts and photocatalysts. J. Porphyr. Phthalocyanines 2004, 8, 1020–1041. [Google Scholar] [CrossRef]
- Nyokong, T. Effects of substituents on the photochemical and photophysical properties of maingroup metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Aoki, I.V.; Guedes, I.G.; Maranhao, S.L. Copper phthalocyanine as corrosion inhibitor for ASTM A606-4 steel in 16% hydrochloric acid. J. Appl. Electrochem. 2002, 32, 915–919. [Google Scholar] [CrossRef]
- Zhao, P.; Liang, Q.; Li, Y. Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/L HCl. Appl. Surf. Sci. 2005, 252, 1596–1607. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Guo, W.; Liu, G.; Ma, H.; Wu, L. Electrochemical and molecular simulation studies on the corrosion inhibition of 5,10,15,20-tetraphenylporphyrin adlayers on iron surface. Appl. Surf. Sci. 2007, 253, 8734–8742. [Google Scholar] [CrossRef]
- Özdemir, O.K.; Aytaç, A.; Atilla, D.; Durmuş, M. Corrosion inhibition of aluminum by novel phthalocyanines in hydrochloric acid solution. J. Mater. Sci. 2011, 46, 752–758. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, K.S.; de Keersmaecker, M.; Elia, A.; Depla, D.; Dubruel, P.; Vandenabeele, P.; van Vlierberghe, S.; Adriaens, A. Adsorption of cobalt (II) 5,10,15,20-tetrakis(2-aminophenyl) porphyrin onto copper substrates: Characterization and impedance studies for corrosion inhibition. Corros. Sci. 2012, 62, 73–82. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. The Porphyrin Handbook: Phthalocyanines: Properties and Materials; Elsevier Science: San Diego, Carlifornia, CA, USA, 2003; Volume 17. [Google Scholar]
- Solomon, M.M.; Umoren, S.A.; Udousoro, I.I.; Udoh, A.P. Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Igwe, I.O.; Ebenso, E.E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halides additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Umoren, S.A.; Li, Y.; Wang, F.H. Effect of polyacrylic acid on the corrosion behaviour of aluminium in sulphuric acid solution. J. Solid State Electr. 2010, 14, 2293–2305. [Google Scholar] [CrossRef]
- Qian, B.; Wang, J.; Zheng, M.; Hou, B. Synergistic effect of polyaspartic acid and iodide ion oncorrosion inhibition of mild steel in H2SO4. Corros. Sci. 2013, 75, 184–192. [Google Scholar] [CrossRef]
- Al Fuhaiman, L.A.; Mustafa, A.A.; Mekhamer, W.K. Polyvinyl pyrrolidone as a green corrosion inhibitor for carbon steel in alkaline solutions containing NaCl. Anti Corros. Method Mater. J. 2013, 60, 26–28. [Google Scholar] [CrossRef]
- Soltani, N.; Behpour, M.; Ghoreishi, S.M.; Naiemi, H. Corrosion inhibition of mild steel in hydrochloric acid solution by some double Schiff bases. Corros. Sci. 2010, 52, 1351–1361. [Google Scholar] [CrossRef]
- Szauer, T.; Brandt, A. Adsorption of oleates of various amines on iron in acidic solution. Electrochim. Acta 1981, 26, 1209–1217. [Google Scholar] [CrossRef]
- Dahmani, M.; Et-Touhami, A.; Al-Deyab, S.S.; Hammouti, B.; Bouyanzer, A. Corrosion inhibition of C38 steel in 1 M HCl: A comparative study of black pepper extract and its isolated piperine. Inter. J. Electrochem. Sci. 2010, 5, 1060–1069. [Google Scholar]
- Hoar, T.P.; Holliday, R.D. The inhibition by quinolines and thioureas of the acid dissolution of mild steel. J. Appl. Chem. 1953, 3, 502–513. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr.; Hurd, R.M. Temperature coefficient of corrosion inhibition. Corrosion 1967, 23, 252–258. [Google Scholar] [CrossRef]
- Benali, O.; Larabi, L.; Merah, S.; Harek, Y. Influence of the methylene blue dye (MBD) on the corrosion inhibition of mild steel in 0.5 M sulphuric acid, Part I: Weight loss and electrochemical studies. J. Mater. Environ. Sci. 2011, 2, 39–48. [Google Scholar]
- Saliyan, V.R.; Adhikari, A.V. Inhibition of corrosion of mild steel in acid media by N′-benzylidene-3-(quinolin-4-ylthio)propanohydrazide. Bull. Mater. Sci. 2007, 31, 699–711. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental and quantum chemical studies of some bis(trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 13282–13299. [Google Scholar] [CrossRef]
- Asegbeloyi, J.N.; Ejikeme, P.M.; Olasunkanmi, L.O.; Adekunle, A.S.; Ebenso, E.E. A novel Schiff base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2′-(ethylenedioxy)diethylamine as potential corrosion inhibitor for mild steel in acidic medium. Materials 2015, 8, 2918–2934. [Google Scholar] [CrossRef]
- Mashuga, M.E.; Olasunkanmi, L.O.; Adekunle, A.S.; Yesudass, S.; Kabanda, M.M.; Ebenso, E.E. Adsorption, thermodynamics and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef]
- Abdallah, M; Asghar, B.H.; Zaafarany, I.; Fouda, A.S. The inhibition of carbon steel corrosion in hydrochloric acid solution using some phenolic compounds. Int. J. Electrochem. Sci. 2009, 51, 282–304. [Google Scholar]
- Foad, E.E.; Abdel Wahaab, S.M.; Deyab, M. Ethoxylated fatty acids as inhibitors for the corrosion of zinc in acid media. Mater. Chem. Phys. 2005, 89, 183–191. [Google Scholar] [CrossRef]
- Gomma, G.K. Corrosion of low carbon steel in sulphuric acid solution in presence of pyrazole-halide mixture. Mater. Chem. Phys. 1998, 55, 241–246. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Ebenso, E.E. The adsorption characteristics and synergistic inhibition between polyethylene glycol and halides ions for the corrosion inhibition of mild steel in acidic medium. Bull. Electrochem. 2006, 22, 155–167. [Google Scholar]
- Ebenso, E.E. Synergistic effect of halide ions on the corrosion inhibition of aluminium in H2SO4 using 2-acetylphenothiazine. Mater. Chem. Phys. 2003, 79, 58–70. [Google Scholar] [CrossRef]
- Ebenso, E.E. Effect of halide ions on the corrosion inhibition of mild steel in H2SO4 using methyl red: Part 1. Bull. Electrochem. 2003, 19, 209–216. [Google Scholar]
- Labari, L.; Harek, Y. Effect of Iodide Ions on Corrosion Inhibition of Mild Steel in 0.5 M H2SO4 by Poly(4-Vinylpyridine) Port. Electrochim. Acta 2004, 22, 227–247. [Google Scholar]
- Labari, L.; Harek, Y.; Traisnel, M.; Mansri, A. Synergistic influence of poly(4-Vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1 M HCl. J. Appl. Electrochem. 2004, 34, 833–839. [Google Scholar]
- Yang, W.; Parr, R.G. Hardness, softness, and the Fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. USA 1985, 82, 6723–6726. [Google Scholar] [CrossRef] [PubMed]
- Udhayakala, P.; Rajendiran, T.V.; Gunasekaran, S. Quantum chemical studies on the efficiencies of vinyl imidazole derivatives as corrosion inhibitors for mild steel. J. Adv. Sci. Res. 2012, 3, 37–44. [Google Scholar]
- Lukovits, I.; Bakó, I.; Shaban, A.; Kálmán, E. Polynomial model of the inhibition mechanism of thiourea derivatives. Electrochim. Acta 1998, 43, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Abreu-Quijano, M.; Palomar-Pardave, M.; Cuan, A.; Romero-Romo, M.; Negron-Silva, G.; Alvarez-Bustamante, R.; Ramirez-Lopez, A.; Herrera-Hernandez, H. Quantum chemical study of 2-mercaptoimidazole. 2-mercapto-5-methylbenzimidazole and 2-mercapto-5-nitrobenzimidazole as corrosion inhibitors for steel. Int. J. Electrochem. Sci. 2011, 6, 3729–3742. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Theoretical organic chemistry. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hehre, W.J.; Random, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Wiberg, K.B. Basis Set Effects on Calculated Geometries: 6-311++G** vs. aug-cc-pVDZ. J. Comput. Chem. 2004, 25, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Brovarets’, O.O.; Zhurakivsky, R.O.; Hovorun, D.M. Does the tautomeric status of the adenine bases change upon the dissociation of the A*.Asyn Topal–Fresco DNA mismatch? A combined QM and QTAIM atomistic insight. Phys. Chem. Chem. Phys. 2014, 16, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Samijlenko, S.P.; Yurenko, Y.P.; Stepanyugin, A.V.; Hovorun, D.M. Tautomeric equilibrium of approach. J. Phys. Chem. B 2010, 114, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Lozynski, M.; Rusinska-Roszak, D. Hydrogen bonding and density functional calculations: The B3LYP approach as the shortest way to MP2 results. J. Phys. Chem. A 1998, 102, 2899–2903. [Google Scholar] [CrossRef]
- Lukovits, I.; Shaban, A.; Kalman, E. Corrosion inhibitors: Quantitative structure activity relationships. Russ. J. Electrochem. 2003, 39, 177–181. [Google Scholar] [CrossRef]
- XLSTAT 2015.1; Data Analysis and Statistics Software for Microsoft Excel; Addinsoft: Paris, France, 2015; http://www.xlstat.com (accessed on 14 August 2015).
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibetsoe, M.; Olasunkanmi, L.O.; Fayemi, O.E.; Yesudass, S.; Ramaganthan, B.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Some Phthalocyanine and Naphthalocyanine Derivatives as Corrosion Inhibitors for Aluminium in Acidic Medium: Experimental, Quantum Chemical Calculations, QSAR Studies and Synergistic Effect of Iodide Ions. Molecules 2015, 20, 15701-15734. https://doi.org/10.3390/molecules200915701
Dibetsoe M, Olasunkanmi LO, Fayemi OE, Yesudass S, Ramaganthan B, Bahadur I, Adekunle AS, Kabanda MM, Ebenso EE. Some Phthalocyanine and Naphthalocyanine Derivatives as Corrosion Inhibitors for Aluminium in Acidic Medium: Experimental, Quantum Chemical Calculations, QSAR Studies and Synergistic Effect of Iodide Ions. Molecules. 2015; 20(9):15701-15734. https://doi.org/10.3390/molecules200915701
Chicago/Turabian StyleDibetsoe, Masego, Lukman O. Olasunkanmi, Omolola E. Fayemi, Sasikumar Yesudass, Baskar Ramaganthan, Indra Bahadur, Abolanle S. Adekunle, Mwadham M. Kabanda, and Eno E. Ebenso. 2015. "Some Phthalocyanine and Naphthalocyanine Derivatives as Corrosion Inhibitors for Aluminium in Acidic Medium: Experimental, Quantum Chemical Calculations, QSAR Studies and Synergistic Effect of Iodide Ions" Molecules 20, no. 9: 15701-15734. https://doi.org/10.3390/molecules200915701





