Gallic Acid Is the Major Active Component of Cortex Moutan in Inhibiting Immune Maturation of Human Monocyte-Derived Dendritic Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Analyses of Cortex Moutan

2.2. Effects of Active Fraction F5 and Its Active Ingredients on Human Monocyte-Derived Dendritic Cells

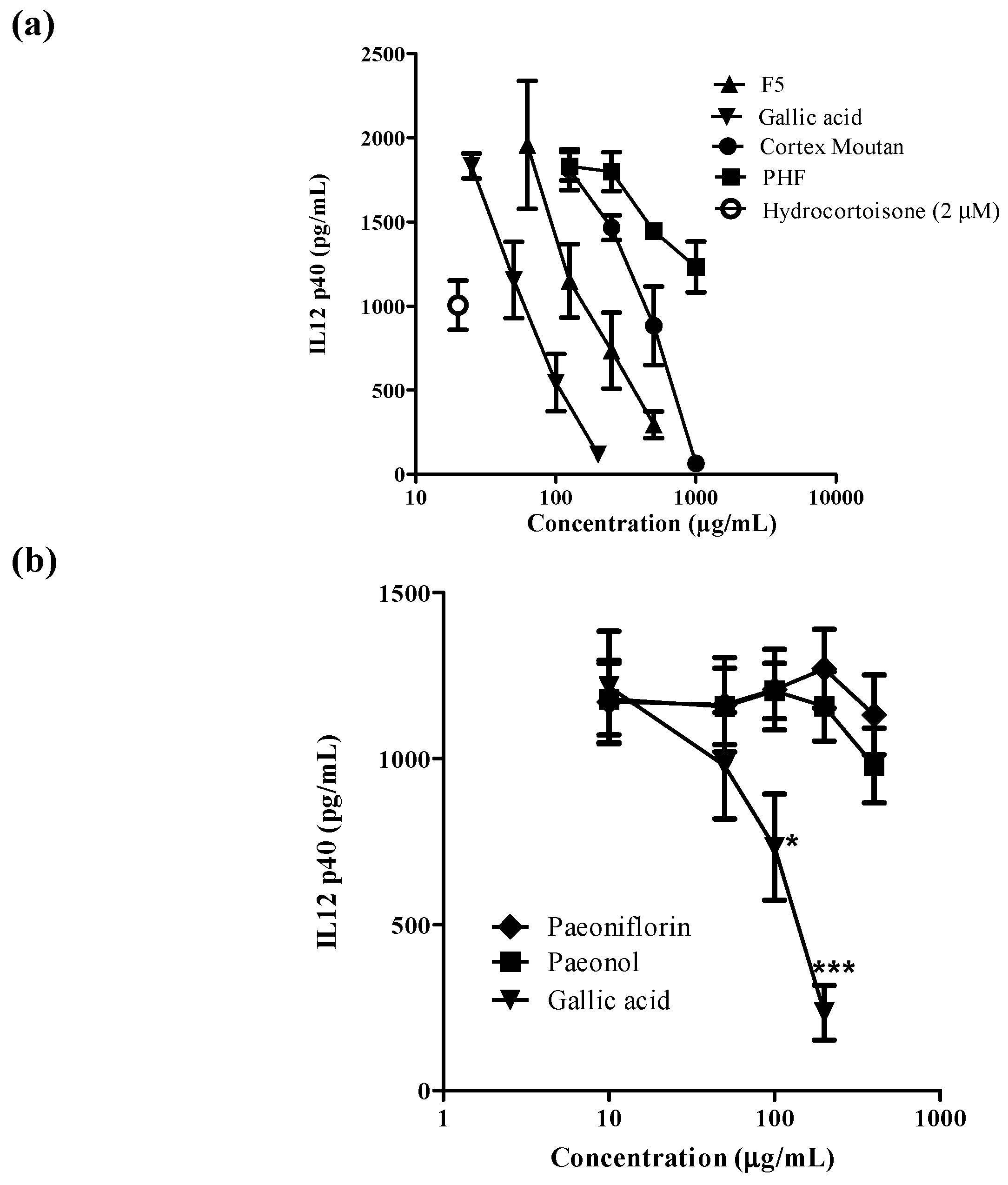
2.3. Effects of Gallic Acid on the Surface Expression of the Functional Markers on Human Monocyte-Derived Dendritic Cells

2.4. Effects of Gallic Acid with Hydrocortisone on the Development of Human Monocyte-Derived Dendritic Cells


3. Experimental Section
3.1. Authentication and Water Extraction of Cortex Moutan
3.2. HSCCC Fractionation and Identification of Active Ingredients from Cortex Moutan
3.3. Effects of the CM HSCCC Fractions on the Production of IL-12p40 from moDC in Vitro
3.4. Cell Isolation and Generation of Dendritic Cells in Vitro
3.5. Flow Cytometric Analysis of Dendritic Cells
3.6. ELISA Assay for Cytokines
3.7. Statistical Analysis
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
Abbreviations
References
- Hon, K.L.; Lau, C.B.; Hui, P.C.; Leung, P.C. Anti-allergic drug discovery in China for eczema: Current methods and future strategies. Expert Opin. Drug Discov. 2013, 8, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Kwiek, B.; Bieber, T. The mode of topical immunomodulators in the immunological network of atopic dermatitis. Clin. Exp. Dermatol. 2005, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Leung, T.F.; Wong, Y.; Lam, W.K.; Guan, D.Q.; Ma, K.C.; Sung, Y.T.; Fok, T.F.; Leung, P.C. A pentaherbs capsule as a treatment option for atopic dermatitis in children: An open-labeled case series. Am. J. Chin. Med. 2004, 32, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Leung, T.F.; Ng, P.C.; Lam, M.C.; Kam, W.Y.; Wong, K.Y.; Lee, K.C.; Sung, Y.T.; Cheng, K.F.; Fok, T.F.; et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: A randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2007, 157, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.F.; Wong, K.Y.; Wong, C.K.; Fung, K.P.; Lam, C.W.; Fok, T.F.; Leung, P.C.; Hon, K.L. In vitro and clinical immunomodulatory effects of a novel Pentaherbs concoction for atopic dermatitis. Br. J. Dermatol. 2008, 158, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Lo, W.; Cheng, W.K.; Leung, T.F.; Chow, C.M.; Lau, C.B.; Fok, T.F.; Ng, P.C.; Leung, P.C. Prospective self-controlled trial of the efficacy and tolerability of a herbal syrup for young children with eczema. J. Dermatol. Treat. 2012, 23, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Hon, K.L.; Leung, P.C.; Sam, S.W.; Fung, K.P.; Lee, M.Y.; Lau, H.Y. Traditional Chinese medicine for atopic eczema: PentaHerbs formula suppresses inflammatory mediators release from mast cells. J. Ethnopharmacol. 2008, 120, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Hu, S.; Chan, B.C.; Wat, E.C.; Lau, C.B.; Hon, K.L.; Fung, K.P.; Leung, P.C.; Hui, P.C.; Lam, C.W.; et al. Anti-inflammatory and anti-allergic activities of Pentaherb formula, Moutan Cortex (Danpi) and gallic acid. Molecules 2013, 18, 2483–2500. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.C.; Vieira, P.L.; Kalinski, P.; Kapsenberg, M.L. Corticosteroids inhibit the production of inflammatory mediators in immature monocyte-derived DC and induce the development of tolerogenic DC3. J. Leukoc. Biol. 1999, 66, 201–204. [Google Scholar] [PubMed]
- Bieber, T.; Novak, N.; Herrman, N.; Koch, S. Role of dendritic cells in atopic dermatitis: An update. Clin. Rev. Allergy Immunol. 2011, 41, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.N.; Wong, M.T.; Zhang, A.L.; Winer, D.; Suhoski, M.M.; Tolentino, L.L.; Gaitan, J.; Davidson, M.G.; Kung, T.H.; Galel, D.M.; et al. TH1, TH2, and TH17 cells instruct monocytes to differentiate into specialized dendritic cell subsets. Blood 2011, 118, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.A.; Park, M.K.; Kim, S.H.; Park, Y.D.; Na, H.J.; Kim, H.M.; Shin, M.K.; Ahn, K.S. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α. Int. Immunopharmacol. 2004, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shin, Y.W.; Bae, E.A.; Han, S.J.; Kim, J.S.; Kang, S.S.; Kim, D.H. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch. Pharm. Res. 2008, 31, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.K.; Shin, T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Radwan, P.; Radwan-Kwiatek, K.; Tabarkiewicz, J.; Radej, S.; Rolinski, J. Enhanced phenotypic and functional maturation of monocyte-derived dendritic cells from patients with active Crohn’s disease and ulcerative colitis. J. Physiol. Pharmacol. 2010, 61, 695–703. [Google Scholar] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, K.; Novak, N. Immunology of atopic eczema: Overcoming the Th1/Th2 paradigm. Allergy 2013, 68, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, H.; Thomson, A.W. Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. 2004, 4, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, L.; Howard, O.M.; Oppenheim, J.J. Dendritic cells as a pharmacological target of traditional Chinese medicine. Cell. Mol. Immunol. 2006, 3, 401–410. [Google Scholar] [PubMed]
- Novak, N.; Haberstok, J.; Kraft, S.; Siekmann, L.; Allam, J.P.; Bieber, T. Standardized extracts from Chinese herbs induce IL-10 production in human monocyte-derived dendritic cells and alter their differentiation in vitro. J. Allergy Clin. Immunol. 2001, 108, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Gessani, S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: Recent advances. Immunobiology 2008, 213, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, J.N.; Brix, S. Isolation of IL-12p70-competent human monocyte-derived dendritic cells. J. Immunol. Methods 2012, 386, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P.; Wieckowski, E.; Muthuswamy, R.; de Jong, E. Generation of stable Th1/CTL-, Th2-, and Th17-inducing human dendritic cells. Methods Mol. Biol. 2010, 595, 117–133. [Google Scholar] [PubMed]
- Kalinski, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997, 159, 28–35. [Google Scholar] [PubMed]
- Gagliardi, M.C.; Sallusto, F.; Marinaro, M.; Langenkamp, A.; Lanzavecchia, A.; de Magistris, M.T. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 2000, 30, 2394–2403. [Google Scholar] [CrossRef]
- Wang, X.S.; Wu, A.Y.; Leung, P.S.; Lau, H.Y. PGE suppresses excessive anti-IgE induced cysteinyl leucotrienes production in mast cells of patients with aspirin exacerbated respiratory disease. Allergy 2007, 62, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Wang, S.S.; Lee, K.K.; Lee, V.W.; Leung, T.F.; Ip, M. Combined antibiotic/corticosteroid cream in the empirical treatment of moderate to severe eczema: Friend or foe? J. Drugs Dermatol. 2012, 11, 861–864. [Google Scholar] [PubMed]
- Shibata, H.; Nakano, T.; Parvez, M.A.; Furukawa, Y.; Tomoishi, A.; Niimi, S.; Arakaki, N.; Higuti, T. Triple combinations of lower and longer alkyl gallates and oxacillin improve antibiotic synergy against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 2218–2220. [Google Scholar] [CrossRef] [PubMed]
- Luca, G.; Basta, G.; Calafiore, R.; Rossi, C.; Giovagnoli, S.; Esposito, E.; Nastruzzi, C. Multifunctional microcapsules for pancreatic islet cell entrapment: Design, preparation and in vitro characterization. Biomaterials 2003, 24, 3101–3114. [Google Scholar] [CrossRef]
- Lee, D.S.; Eom, S.H.; Kim, Y.M.; Kim, H.S.; Yim, M.J.; Lee, S.H.; Kim, D.H.; Je, J.Y. Antibacterial and synergic effects of gallic acid-grafted-chitosan with beta-lactams against methicillin-resistant Staphylococcus aureus (MRSA). Can. J. Microbiol. 2014, 60, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.C.; Wang, W.Y.; Kan, C.W.; Ng, F.S.; Wat, E.; Zhang, V.X.; Chan, C.L.; Lau, C.B.; Leung, P.C. Microencapsulation of traditional Chinese herbs—PentaHerbs extracts and potential application in healthcare textiles. Colloids Surf. 2013, 111, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.C.; Wang, W.Y.; Kan, C.W.; Zhou, C.E.; Ng, F.S.; Wat, E.; Zhang, V.X.; Chan, C.L.; Lau, C.B.; Leung, P.C. Preparation and characterisation of chitosan microcapsules loaded with Cortex Moutan. Int. J. Biol. Macromol. 2013, 55, 32–38. [Google Scholar] [CrossRef] [PubMed]
- State Pharmacopoeia Commission of China. 2010 Pharmacopoeia of the People’s Republic of China; China Medico-Pharmaceutical Science & Technology Publishing House: Beijing, China, 2010; Volume 1. [Google Scholar]
- Sample Availability: Samples of the Pentaherb formula and Cortex Moutan are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, B.C.L.; Li, L.F.; Hu, S.Q.; Wat, E.; Wong, E.C.W.; Zhang, V.X.; Lau, C.B.S.; Wong, C.K.; Hon, K.L.E.; Hui, P.C.L.; et al. Gallic Acid Is the Major Active Component of Cortex Moutan in Inhibiting Immune Maturation of Human Monocyte-Derived Dendritic Cells. Molecules 2015, 20, 16388-16403. https://doi.org/10.3390/molecules200916388
Chan BCL, Li LF, Hu SQ, Wat E, Wong ECW, Zhang VX, Lau CBS, Wong CK, Hon KLE, Hui PCL, et al. Gallic Acid Is the Major Active Component of Cortex Moutan in Inhibiting Immune Maturation of Human Monocyte-Derived Dendritic Cells. Molecules. 2015; 20(9):16388-16403. https://doi.org/10.3390/molecules200916388
Chicago/Turabian StyleChan, Ben Chung Lap, Long Fei Li, Shui Qing Hu, Elaine Wat, Eric Chun Wai Wong, Vanilla Xin Zhang, Clara Bik San Lau, Chun Kwok Wong, Kam Lun Ellis Hon, Patrick Chi Leung Hui, and et al. 2015. "Gallic Acid Is the Major Active Component of Cortex Moutan in Inhibiting Immune Maturation of Human Monocyte-Derived Dendritic Cells" Molecules 20, no. 9: 16388-16403. https://doi.org/10.3390/molecules200916388





