Artemisinin and Its Derivatives as a Repurposing Anticancer Agent: What Else Do We Need to Do?
Abstract
:1. Introduction
2. Foregoing Researches on the Anticancer Effect of Artemisinin and Its Derivates
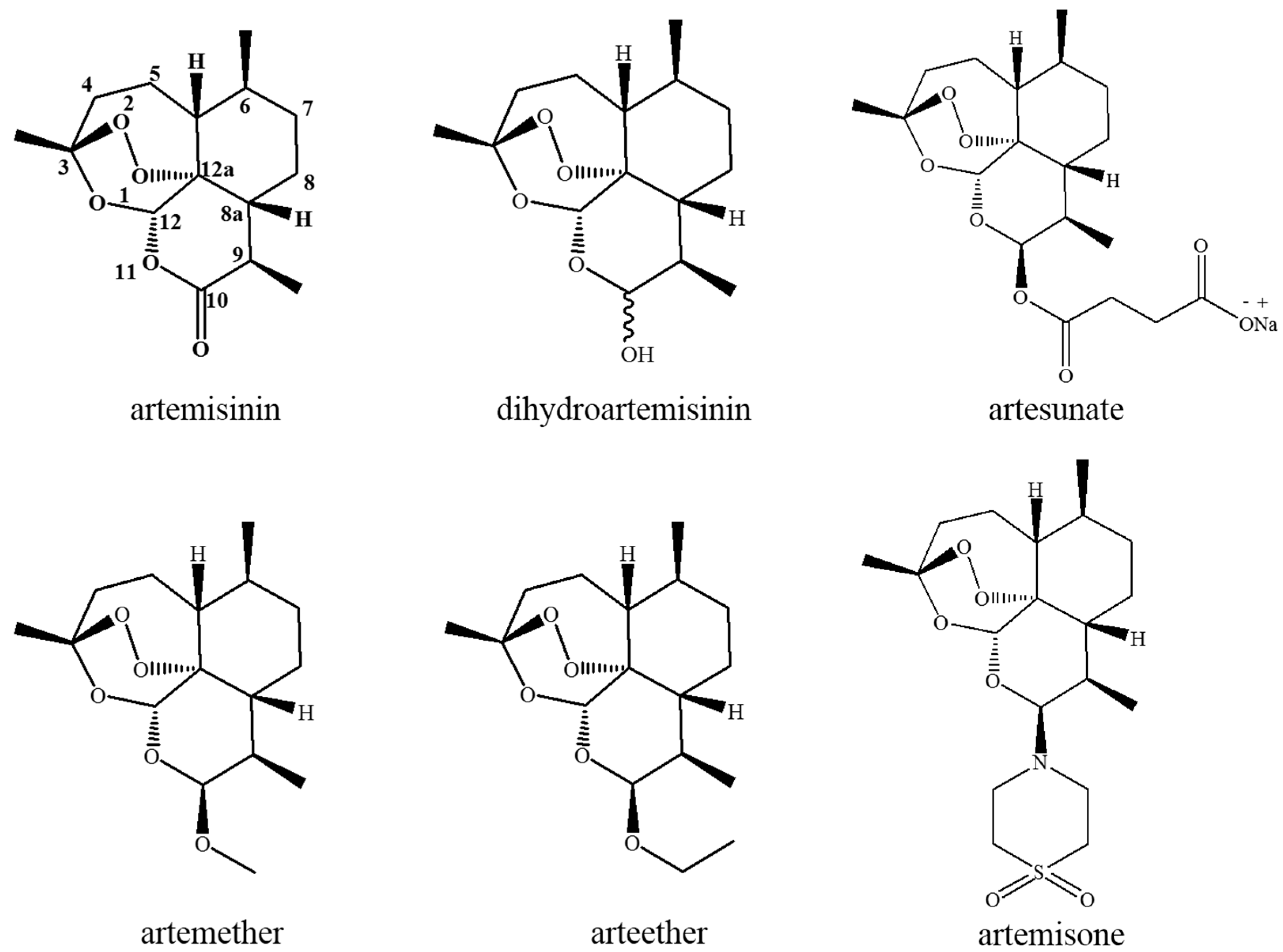
2.1. Chemistry of Artemisinin and Its Derivates
2.2. Known Anticancer Activity and Mechanism of Action
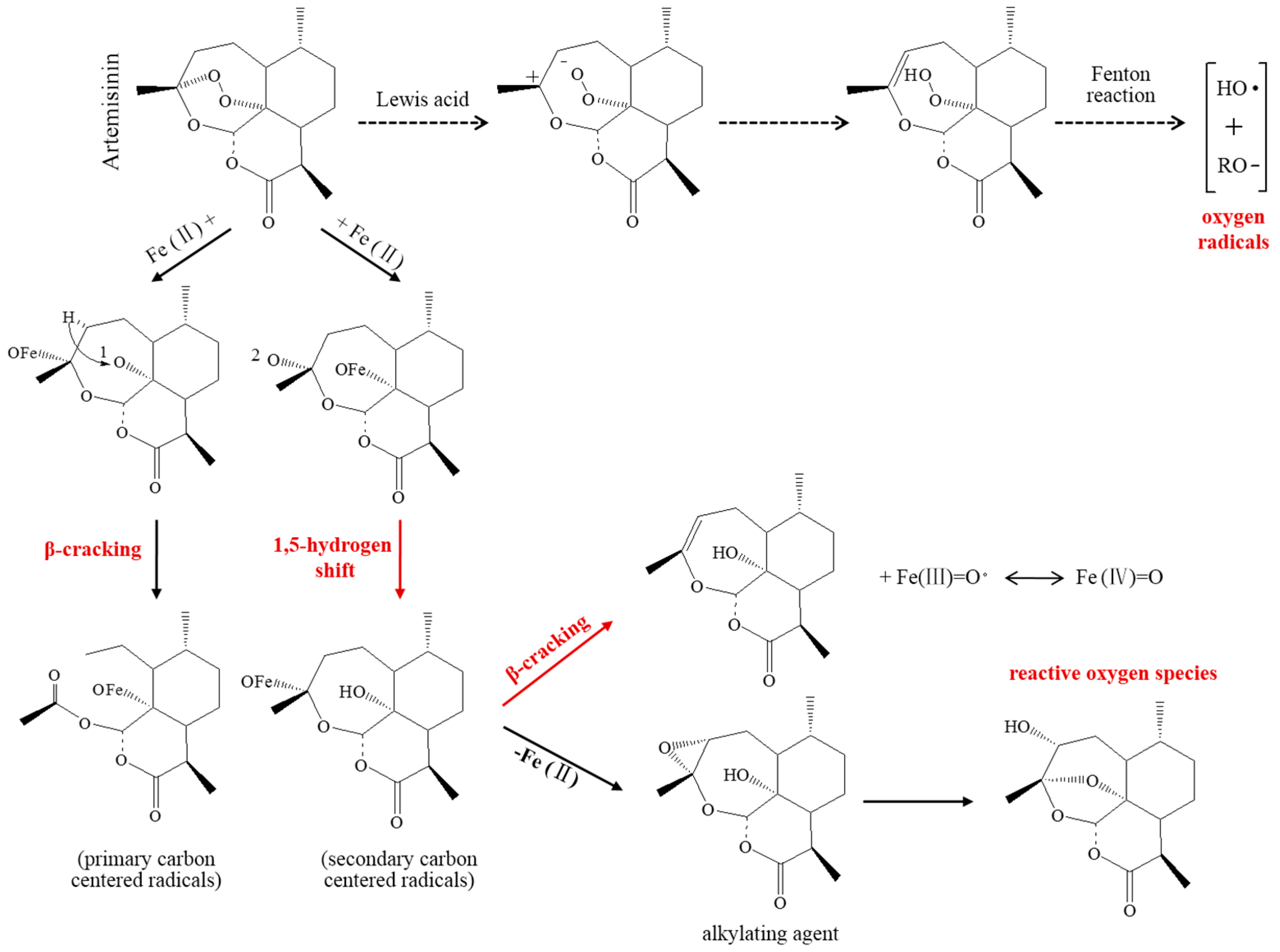
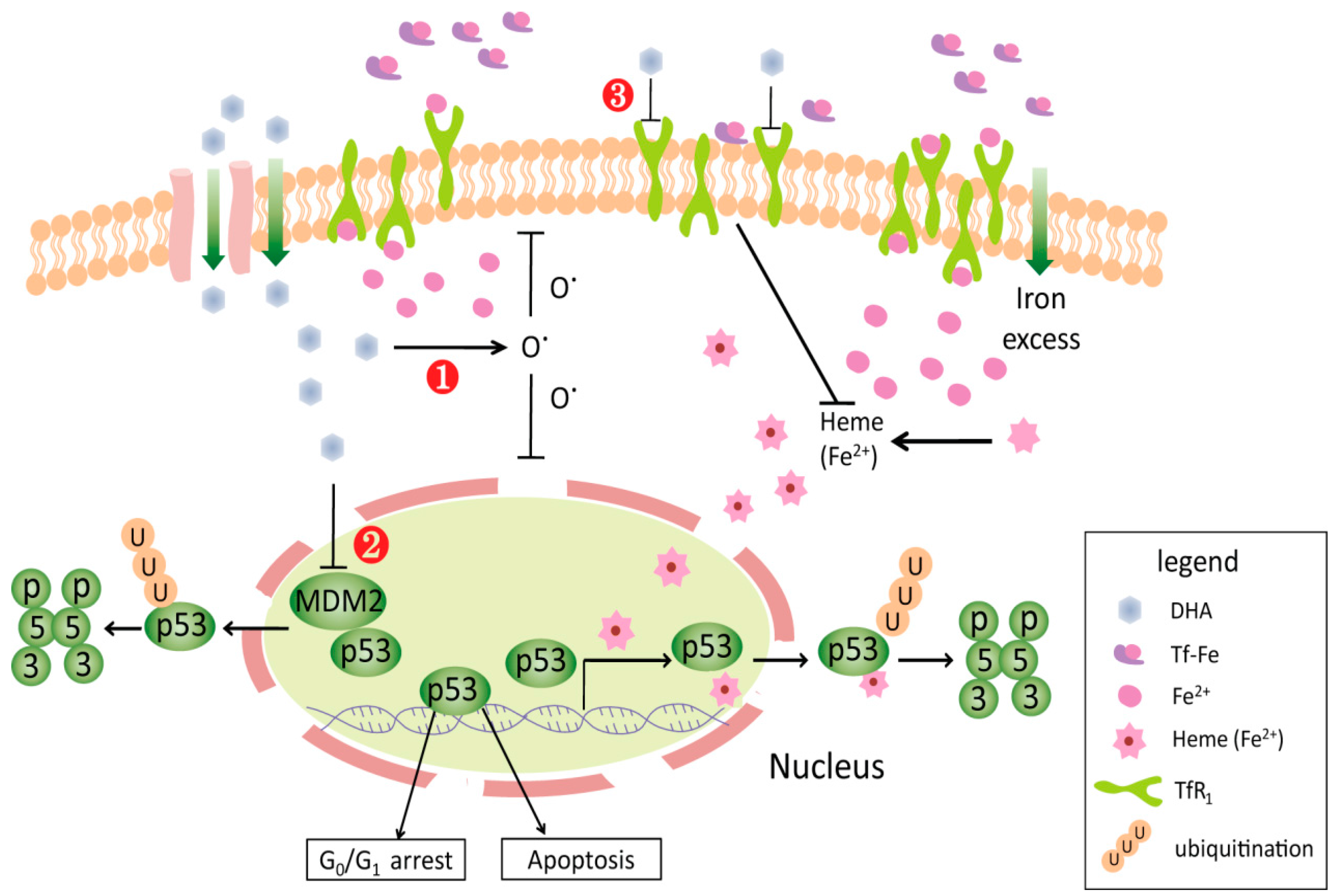
2.2.1. Toxic-Free Radicals Generated by Endoperoxide Moiety
2.2.2. Cell Cycle Arrest
2.2.3. Induction of Apoptosis
2.2.4. Inhibition of Tumor Angiogenesis
2.3. Safety Investigation
2.4. Clinical Experience
3. Future Direction
3.1. Further Investigations on Mechanism of Action
3.2. Exploration of an Efficient and Specific Drug Delivery System
3.3. Strategy in Clinical Application
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Boichuk, S.; Lee, D.J.; Mehalek, K.R.; Makielski, K.R.; Wozniak, A.; Seneviratne, D.S.; Korzeniewski, N.; Cuevas, R.; Parry, J.A.; Brown, M.F.; et al. Unbiased compound screening identifies unexpected drug sensitivitie sand novel treatment options for gastrointestinal stromal tumors. Cancer. Res. 2014, 74, 1200–1213. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.E.; Rothwell, P.M. Aspirin in gastrointestinal oncology: New data on an old friend. Curr. Opin. Oncol. 2014, 26, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Price, J.F.; Fowkes, F.G.; Zanchetti, A.; Roncaglioni, M.C.; Tognoni, G.; Lee, R.; Belch, J.F.; Wilson, M.; Mehta, Z.; et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012, 379, 1602–1612. [Google Scholar] [CrossRef]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Yiannakopoulou, E.C. Aspirin and NSAIDs for breast cancer chemoprevention. Eur. J. Cancer Prev. 2015, 24, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chhipa, R.R.; Pooya, S.; Wortman, M.; Yachyshin, S.; Chow, L.M.; Kumar, A.; Zhou, X.; Sun, Y.; Quinn, B.; et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc. Natl. Acad. Sci. USA 2014, 111, E435–E444. [Google Scholar] [CrossRef] [PubMed]
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Asselbergs, E.; Zweegman, S.; van der, H.B.; Kersten, M.J.; Vellenga, E.; van Marwijk-Kooy, M.; Broyl, A.; deWeerdt, O.; Lonergan, S.; et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood 2015, 125, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ng, J.; Christos, P.J.; Goldenberg, A.; Sparano, J.; Sung, M.W.; Hochster, H.S.; Muggia, F.M. Chronic thalidomide and chemoembolization for hepatocellular carcinoma. Oncologist 2014, 19, 1229–1330. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P. Repurposing drugs in oncology (ReDO)-cimetidine as an anti-cancer agent. Ecancermedicalscience 2014, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Sidner, R.D. Antitumour effect of cimetidine. Lancet 1979, 1, 882–893. [Google Scholar] [CrossRef]
- Zhang, X.W.; Yan, X.J.; Zhou, Z.R.; Yang, F.F.; Chen, S.J.; Chen, Z. Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science 2010, 328, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, E.; Kosciuczuk, E.M.; Serrano, R.; Nanavati, D.; Swindell, E.P.; Viollet, B.; O’Halloran, T.V.; Altman, J.K.; Platanias, L.C. Direct binding of arsenic trioxide to AMPK and generation of inhibitory effects on acute myeloid leukemia precursors. Mol. Cancer Ther. 2015, 14, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Collaboration Research Group for Qinghaosu. A novel kind of sesquiterpene lactone-artemisinin. Chin. Sci. Bullet. 1977, 22, 142. [Google Scholar] [CrossRef]
- Liu, J.M.; Ni, M.Y.; Zhou, W.S. Structure and reaction of qinghaosu (Arteannuin). Acta. Chimica Sinica 1979, 37, 129–141. [Google Scholar]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Kakar, Q.; Sheikh, S.; Ahmed, I.; Khan, M.A.; Jamil, M.; ElMohammady, H.; Warsame, M. Efficacy of artemisinin-based combination therapies for the treatment of falciparum malaria in Pakistan (2007–2015): In vivo response and dhfr and dhps mutations. Acta. Trop. 2016, 164, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Woerdenbag, H.J.; Moskal, T.A.; Pras, N.; Malingré, T.M.; El-Feraly, F.S.; Kampinga, H.H.; Konings, A.W. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J. Nat. Prod. 1993, 56, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.E.; Volm, M.; Tew, K.D.; et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, D.; Zhang, R.; Wang, H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008, 14, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, M.; Zhang, R.; Wang, H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell. Mol. Med. 2009, 13, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Hu, Y.; Li, Z.; Wang, P.; Xue, Y.X.; Yao, Y.L.; Yu, B.; Liu, Y.H. Artemether combined with shRNA interference of vascular cell adhesion molecule-1 significantly inhibited the malignant biological behavior of human glioma cells. PLoS ONE 2013, 8, e60834. [Google Scholar] [CrossRef] [PubMed]
- Alcntara, D.D.; Ribeiro, H.F.; Cardoso, P.C.; Araújo, T.M.; Burbano, R.R.; Guimarães, A.C.; Khayat, A.S.; Oliveira, B.M. In vitro evaluation of the cytotoxic and genotoxic effects of artemether, an antimalarial drug, in a gastric cancer cell line (PG100). J. Appl. Toxicol. 2013, 33, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Azimi Mohamadabadi, M.; Hassan, Z.M.; Zavaran, H.A.; Gholamzad, M.; Noori, S.; Mahdavi, M.; Maroof, H. Arteether exerts antitumor activity and reduces CD4+CD25+FOXP3+T-reg cells in vivo. Iran J. Immunol. 2013, 10, 139–149. [Google Scholar] [PubMed]
- Ericsson, T.; Blank, A.; von Hagens, C.; Ashton, M.; Abelö, A. Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur. J. Clin. Pharmacol. 2014, 70, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Deng, D.A.; Zhang, S.G.; Xu, R.S. Structure of artemisilactone. Acta Chimica Sinica 1984, 48, 937–939. [Google Scholar]
- Gravett, A.M.; Liu, W.M.; Krishna, S.; Chan, W.C.; Haynes, R.K.; Wilson, N.L.; Dalgleish, A.G. In vitro study of the anti-cancer effects of artemisone alone or in combination with other chemotherapeutic agents. Cancer Chemother Pharmacol. 2011, 67, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tan, X.J. Research advance in antitumor activities of artemisinin and its derivatives. Zhong Guo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 466–471. [Google Scholar]
- Chow, L.M.; Chan, T.H. Novel classes of dimer antitumour drug candidates. Curr. Pharm. Des. 2009, 15, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Jia, G.; Chen, Z.X.; Wang, Y.W.; Mu, M.; Wang, S.J.; Pan, S.H.; Gao, Y.; Jian, H.C.; Dong, D.L.; et al. Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5. PLoS ONE 2012, 7, e37222. [Google Scholar] [CrossRef]
- Berdelle, N.; Nikolova, T.; Quiros, S.; Efferth, T.; Kaina, B. Artesunateinduces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol. Cancer Ther. 2011, 10, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.E.; Copple, I.M.; Maggs, J.L.; O’Neill, P.M.; Park, B.K. The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J. Biol. Chem. 2011, 286, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr. Drug Targets 2006, 7, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Gallis, B.; Takatani-Nakase, T.; Oh, S.; Lacoste, E.; Singh, N.P.; Goodlett, D.R.; Tanaka, S.; Futaki, S.; Lai, H.; Sasaki, T. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009, 274, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Meng, L.H.; Cai, Y.J.; Chen, Q.; Tong, L.J.; Lin, L.P.; Ding, J. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol. Ther. 2008, 7, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.W.; Singh, N.P.; Lai, H.C. Cytotoxicity of dihydroartemisinin toward Molt-4 cells attenuated by N-tert-butyl-α-phenylnitrone and deferoxamine. Anticancer Res. 2013, 33, 4389–4393. [Google Scholar] [PubMed]
- Jiang, Z.; Chai, J.; Chuang, H.H.; Li, S.; Wang, T.; Cheng, Y.; Chen, W.; Zhou, D. Artesunate induces G0/G1 cell cycle arrest and iron-mediated mitochondrial apoptosis in A431 human epidermoid carcinoma cells. Anticancer Drugs. 2012, 23, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gong, R.; Shi, X.; Yang, D.; Zhang, G.; Lu, A.; Yue, J.; Bian, Z. Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget 2016. [Google Scholar] [CrossRef]
- Willoughby, J.A., Sr.; Sundar, S.N.; Cheung, M.; Tin, A.S.; Modiano, J.; Firestone, G.L. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J. Biol. Chem. 2009, 284, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shou, L.M.; Lin, F.; Duan, W.M.; Wu, M.Y.; Xie, X.; Xie, Y.F.; Li, W.; Tao, M. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Eur. J. Haematol. 2013, 91, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Steinbrück, L.; Pereira, G.; Efferth, T. Effects of artesunate on cytokinesis and G0/M cell cycle progression of tumour cells and budding yeast. Cancer Geno. Proteom. 2010, 7, 337–346. [Google Scholar]
- Wang, S.J.; Gao, Y.; Chen, H.; Kong, R.; Jiang, H.C.; Pan, S.H.; Xue, D.B.; Bai, X.W.; Sun, B. Dihydroartemisinin inactivates NF-κB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 2010, 293, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Li, C.; Li, C.; Wei, L.; Li, L.; Zhang, Y.; Yao, Y.; Gu, X.; Cai, W.; Yang, Z.; et al. The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, F.; Cheng, Z.; Yang, X.; Wang, S.; Geng, F.; Pan, L. Effect ofartesunateon inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NF-κB p65. Int. J. Hematol. 2009, 90, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.P.; Zhang, P.Z. Artesunatemitigates proliferation of tumor cells by alkylating heme-harboring nitric oxide synthase. Nitric Oxide 2011, 24, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.D.; Tan, S.H.; Ng, S.; Shi, Y.; Zhou, J.; Tan, K.S.; Wong, W.S.; Shen, H.M. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J. Biol. Chem. 2014, 289, 33425–33441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Pan, W.; Wang, X.P.; Chen, T.S. Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells. J. Cell. Physiol. 2012, 227, 3778–3786. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Stein, H.A.; Turschner, S.; Toegel, I.; Mora, R.; Jennewein, N.; Efferth, T.; Eils, R.; Brady, N.R. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J. Biol. Chem. 2011, 286, 6587–6601. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, Y.C.; Pei, L.B.; Shi, L.L.; Yan, J.L.; Ma, X.H. Anti-tumor effects of dihydroartemisinin on human osteosarcoma. Mol. Cell. Biochem. 2011, 351, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Holien, T.; Olsen, O.E.; Misund, K.; Hella, H.; Waage, A.; Rø, T.B.; Sundan, A. Lymphoma and myeloma cells are highly sensitive to growth arrest and apoptosis induced by artesunate. Eur. J. Haematol. 2013, 91, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ge, C.M.; Meng, Q.H.; Cao, J.P.; Tong, J.; Fan, S.J. Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol. Sin. 2007, 28, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, B.; Wang, S.; Pan, S.; Gao, Y.; Bai, X.; Xue, D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: Involvement of cell cycle arrest and inactivation of nuclear factor-κB. J. Cancer Res. Clin. Oncol. 2010, 136, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Meng, L.H.; Shankavaram, U.T.; Zhu, C.H.; Tong, L.J.; Chen, G.; Lin, L.P.; Weinstein, J.N.; Ding, J. Dihydroartemisinin accelerates c-MYC oncoprotein degradation and induces apoptosis in c-MYC-overexpressing tumor cells. Biochem. Pharmacol. 2010, 80, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.G.; Fernandez-Dolon, M.; Sanchez-Vicente, L.; Maestre, A.D.; Gomez-San, M.A.B.; Alvarez, M.; Serrano, M.A.; Jansen, H.; Efferth, T.; Marin, J.J.; et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg. Med. Chem. 2013, 21, 4432–4441. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castaño, B.; Macias, R.I.; Briz, O.; Marin, J.J. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral. Res. 2005, 68, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Chen, W.; Yu, B.; Zhang, C.; Zhang, Y.; Qi, H. Calcium and survivin are involved in the induction of apoptosis by dihydroartemisinin in human lung cancer SPC-A-1 cells. Methods Find Exp. Clin. Pharmacol. 2007, 29, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhang, W.; Chu, D.; Liu, T.; Xie, Y.; Fu, E.; Jin, F. The role of calcium, P38 MAPK in dihydroartemisinin-induced apoptosis of lung cancer PC-14 cells. Cancer Chemother Pharmacol. 2008, 61, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Kong, R.; Ma, Z.B.; Han, B.; Wang, Y.W.; Pan, S.H.; Li, Y.H.; Sun, B. The activation of c-Jun NH2-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2014, 33, 8. [Google Scholar] [CrossRef] [PubMed]
- Njokah, M.J.; Kang’ethe, J.N.; Kinyua, J.; Kariuki, D.; Kimani, F.T. In vitro selection of Plasmodium falciparum Pfcrt and Pfmdr1 variants by artemisinin. Malar. J. 2016, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Sun, B.; Cheng, Z.X.; Zhou, H.X.; Gao, Y.; Kong, R.; Chen, H.; Jiang, H.C.; Pan, S.H.; Xu, D.B.; et al. Dihydroartemisin in inhibits angiogenesis in pancreatic cancer by targeting the NF-κB pathway. Cancer Chemother. Pharmacol. 2011, 68, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhou, X.; Li, C.; Yan, S.; Deng, X.; Cao, Z.; Li, L.; Tang, B.; Allen, T.D.; Liu, J. Dihydroartemisin in targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis. Cancer Biol. Ther. 2014, 15, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dong, F.; Hou, Y.; Cai, W.; Zhou, X.; Huang, A.L.; Yang, M.; Allen, T.D.; Liu, J. Dihydroartemisin in inhibits vascular endothelial growth factor-induced endothelial cell migration by a p38 mitogen-activated protein kinase- independent pathway. Exp. Ther. Med. 2014, 8, 1707–1712. [Google Scholar] [PubMed]
- Saeed, M.E.; Kadioglu, O.; Seo, E.J.; Greten, H.J.; Brenk, R.; Efferth, T. Quantitative structure-activity relationship and molecular docking of artemisinin derivatives to vascular endothelial growth factor receptor 1. Anticancer Res. 2015, 35, 1929–1934. [Google Scholar] [PubMed]
- Chen, H.; Shi, L.; Yang, X.; Li, S.; Guo, X.; Pan, L. Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int. J. Hematol. 2010, 92, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Konkimalla, V.B.; McCubrey, J.A.; Efferth, T. The role of downstream signaling pathways of the epidermal growth factor receptor for artesunate’s activity in cancer cells. Curr. Cancer Drug Targets. 2009, 9, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yao, Q.; Zhang, A.M.; Lin, S.; Wang, X.X.; Wu, L.; Sun, J.G.; Chen, Z.T. The effects of artesunate on the expression of EGFR and ABCG2 in A549 human lung cancer cells and a xenograft model. Molecules 2011, 16, 10556–10569. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Zhang, H.D.; Yuan, S.J.; Yang, D.X.; Wang, L.; Sun, Z.X. Differential sensitivity of colorectal cancer cell lines to artesunate is associated with expression of β-catenin and E-cadherin. Eur. J. Pharmacol. 2008, 588, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krusche, B.; Arend, J.; Efferth, T. Synergistic inhibition of angiogenesis by artesunate and captopril in vitro and in vivo. Evid. Based Compl. Alternat. Med. 2013, 2013, 454783. [Google Scholar] [CrossRef]
- Ba, Q.; Duan, J.; Tian, J.Q.; Wang, Z.L.; Chen, T.; Li, X.G.; Wu, S.J.; Xiang, L.; Li, J.Q.; Chu, R.A.; et al. Dihydroartemisinin promotes angiogenesis during the early embryonic development of zebrafish. Acta Pharmacol. Sin. 2013, 34, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Weina, P.J. Severe embryotoxicity of artemisinin derivatives in experimental animals, but possibly safe in pregnant women. Molecules 2009, 15, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Boareto, A.C.; Dalsenter, P.R. Clinical and non-clinical safety of artemisinin derivatives in pregnancy. Reprod. Toxicol. 2016, 65, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Rutteman, G.R.; Erich, S.A.; Mol, J.A.; Spee, B.; Grinwis, G.C.; Fleckenstein, L.; London, C.A.; Efferth, T. Safety and efficacy field study of artesunate for dogs with non-resectable tumours. Anticancer Res. 2013, 33, 1819–1827. [Google Scholar] [PubMed]
- Schmuck, G.; Roehrdanz, E.; Haynes, R.K.; Kahl, R. Neurotoxic mode of action of artemisinin. Antimicrob. Agents Chemother. 2002, 46, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. EBioMedicine 2015, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.G.; Dieckmann, D.; Efferth, T.; Schultz, E.S.; Funk, J.O.; Baur, A.; Schuler, G. Artesunate in the treatment of metastatic uveal melanoma-first experiences. Oncol. Rep. 2005, 14, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Ren, J. Application Value of Interventional Ultrasound in Treatment of Primary Hepatic. Mod. Diag. Treat. 2007, 18, 121. [Google Scholar]
- Zhang, Z.Y.; Yu, S.Q.; Miao, L.Y.; Huang, X.Y.; Zhang, X.P.; Zhu, Y.P.; Xia, X.H.; Li, D.Q. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2008, 6, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Panwar, V.K. Case report of a pituitary macro adenoma treated with artemether. Integr. Cancer Ther. 2006, 5, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Peng, D.; Wang, Y.; Ren, Q.; Guo, Y. The production and exportation of artemisinin-derived drugs in China: current status and existing challenges. Malar. J. 2016, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Wang, S.; Xuan, Z.; Li, D.; Wu, Y.; Shang, Y.; Kong, X.; et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014, 7, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Ba, Q.; Zhou, N.; Duan, J.; Chen, T.; Hao, M.; Yang, X.; Li, J.; Yin, J.; Chu, R.; Wang, H. Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PLoS ONE 2012, 7, e42703. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Lam, E.; Roos, W.P.; Zdzienicka, M.Z.; Kaina, B.; Efferth, T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res. 2008, 68, 4347–4351. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ba, Q.; Yue, Q.; Li, J.; Li, J.; Chu, R.; Wang, H. Artemisinin rewires the protein interaction network in cancer cells: Network analysis, pathway identification, and target prediction. Mol. Biosyst. 2013, 9, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Ochiuz, L.; Grigoras, C.; Popa, M.; Stoleriu, I.; Munteanu, C.; Timofte, D.; Profire, L.; Grigoras, A.G. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules 2016, 21, 858. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, S.E.; Su, Z.; Shi, Y.; Li, S.; Xiao, Y.; Ping, Q. Preparation and optimization of transferrin-modified- artemether lipid nanospheres based on the orthogonal design of emulsion formulation and physically electrostatic adsorption. Int. J. Pharm. 2013, 452, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Faezizadeh, Z.; Mesbah-Namin, S.A.; Saravani, R. Preparation, characterization and in vitro efficacy of magnetic nanoliposomes containing the artemisinin and transferrin. DARU J. Pharm. Sci. 2014, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shen, X.; Zhao, C.; Qin, X.; Liu, H.; Huang, L.; Qiu, Z.; Liu, Y. In vivo study of effects of artesunate nanoliposomes on human hepatocellular carcinoma xenografts in nude mice. Drug Deliv. 2013, 20, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Liang, W.; Huang, Y. An injectable hybrid nanoparticle-in-oil-in-water submicron emulsion for improved delivery of poorly soluble drugs. Nanoscale Res. Lett. 2012, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Wang, X.; Zhao, X.; Ren, T.; Wang, F.; Yu, B. Enhanced delivery of artemisinin and its analogues to cancer cells by their adducts with human serum transferrin. Int. J. Pharm. 2014, 467, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Ma, J.; Zhang, H.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Z.; Wang, H.B.; Zhou, J.J.; Zhang, W.J.; Chen, Q.W. Multifunctional mesoporous nanoparticles as pH-responsive Fe2+ reservoirs and artemisinin vehicles for synergistic inhibition of tumor growth. Biomaterials 2014, 35, 6498–6507. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Tran, T.H.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly-d,l-lactide-co-glycolide (PLGA) nanoparticles. Arch. Pharm. Res. 2015, 38, 716–724. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Malhotra, M.; O’Mahony, A.M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticlesand the Blood-BrainBarrier: Advancing from in-Vitro Models towards Therapeutic Significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, P.; Shalviri, A.; Henderson, J.T.; He, C.; Foltz, W.D.; Prasad, P.; Brodersen, P.M.; Chen, Y.; DaCosta, R.; et al. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano. 2014, 8, 9925–9940. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L. Creation and revelation: Two different routes to advancement in the biomedical sciences. Nat. Med. 2007, 13, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Investig. New Drugs. 2013, 31, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Bhatia, S.; Garg, A.; Sharma, A.; Choudhary, V. Phase 0 clinical trials in oncology new drug development. Perspect. Clin. Res. 2011, 2, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, W.; Li, B.; Yao, Q.; Dong, J.; Cen, Y.; Pan, X.; Li, J.; Zheng, J.; Pang, X.; et al. Artesunate enhances radio sensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int. Immunopharmacol. 2011, 11, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
| Agents | Purpose | Repurposing Cancer Types | References |
|---|---|---|---|
| Aspirin | Relieve pain, reduce fever, prevent blood from clotting | Gastrointestinal oncology, estrogen receptor-negative breast cancer | [2,3,4,5] |
| Metformin | Type 2 diabetes mellitus | Colorectal, breast, prostate colon, brain and non-small cell lung cancer | [6,7] |
| Thalidomide | Leprosy | Multiple myeloma, hepatocellular carcinoma | [8,9] |
| Cimitidine | Peptic ulcer | Colorectal cancer, melanoma, renal cell carcinoma, pancreatic carcinoma, Gastric carcinoma | [10,11] |
| Arsenic | Lung diseases and psoriasis | Leukemia | [12,13] |
| Compounds | Developed Formulations | Reference |
|---|---|---|
| Artemisinin | Magnetic nanoliposomes, adducts with human serum transferring, LyP-1 modification to polymeric micelles, multifunctional mesoporous nanoparticles | [89,92,93,94] |
| Artemether | Lipid nanospheres | [88] |
| Artesunate | Nanoliposomes, PLGA nanoparticles | [90,95] |
| Dihydroartemisinin | Nanoparticles-in-oil-in-water submicron emulsion | [91] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Q.; Wu, J.; Wang, M.; Yu, J. Artemisinin and Its Derivatives as a Repurposing Anticancer Agent: What Else Do We Need to Do? Molecules 2016, 21, 1331. https://doi.org/10.3390/molecules21101331
Li Z, Li Q, Wu J, Wang M, Yu J. Artemisinin and Its Derivatives as a Repurposing Anticancer Agent: What Else Do We Need to Do? Molecules. 2016; 21(10):1331. https://doi.org/10.3390/molecules21101331
Chicago/Turabian StyleLi, Zhe, Qin Li, Jun Wu, Manyuan Wang, and Junxian Yu. 2016. "Artemisinin and Its Derivatives as a Repurposing Anticancer Agent: What Else Do We Need to Do?" Molecules 21, no. 10: 1331. https://doi.org/10.3390/molecules21101331





