Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber
Abstract
:1. Introduction
2. The Role of Solvent-Free Synthesis in Coordination Chemistry
3. Mechanochemical Synthesis of Coordination Polymers
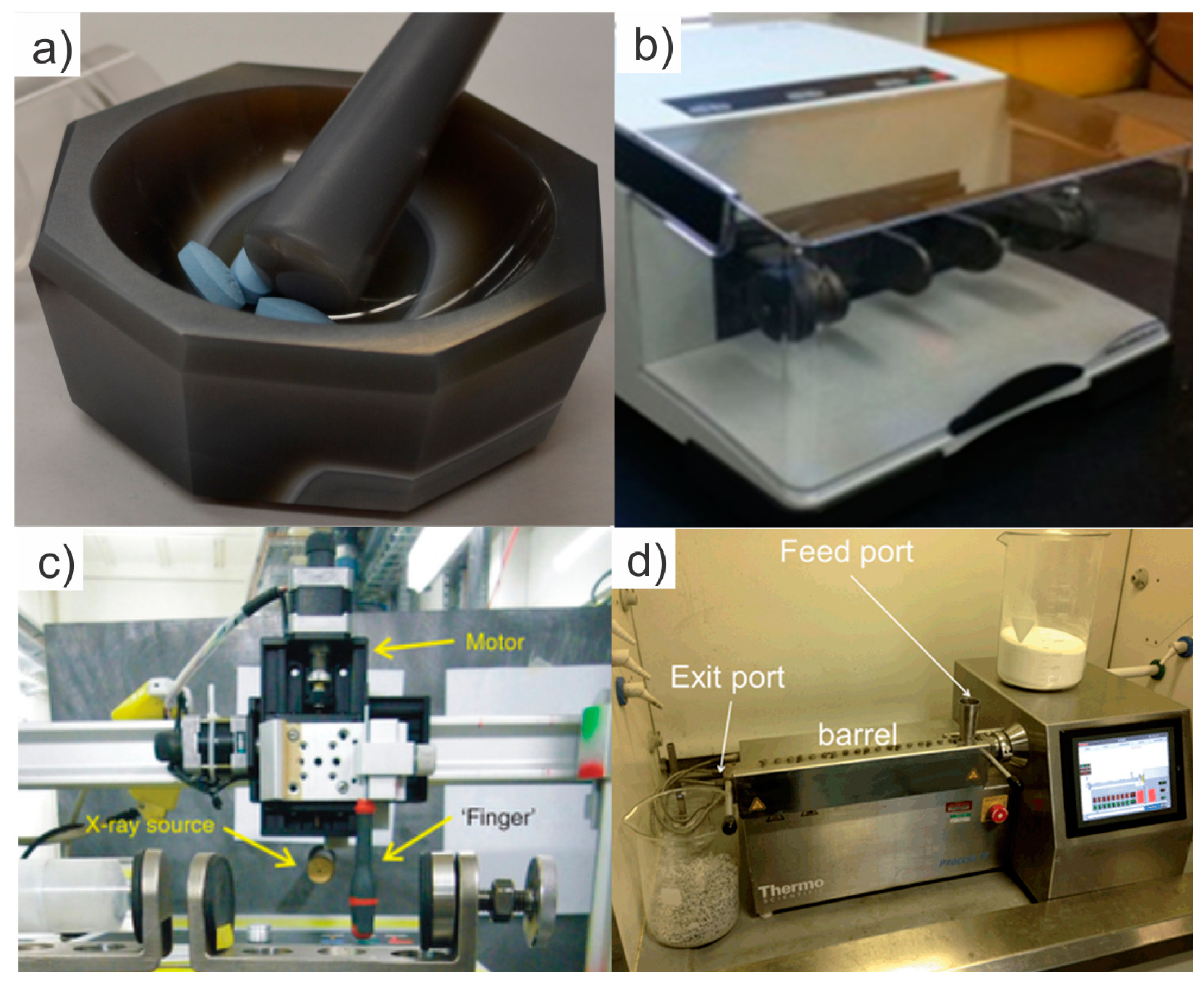
3.1. Mechanochemistry, History, Scope and Benefits
3.2. Mechanochemical Synthesis of Coordination Polymers by Neat Grinding
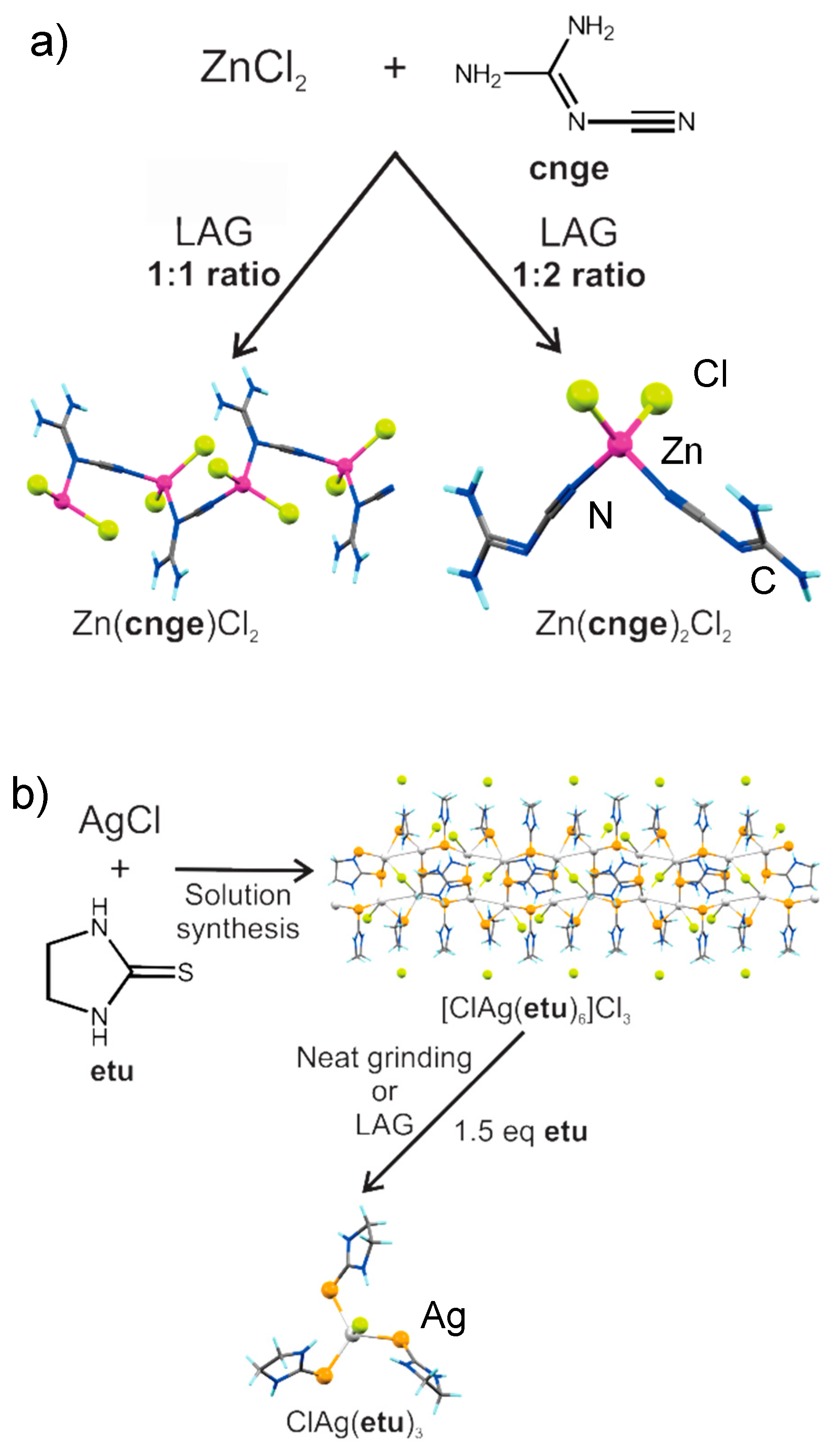
3.3. Mechanochemical Synthesis of Coordination Polymers by Liquid-Assisted Grinding (LAG)
3.4. Mechanochemical Screening for Metal-Based Derivatives of Active Pharmaceutical Ingredients (APIs)
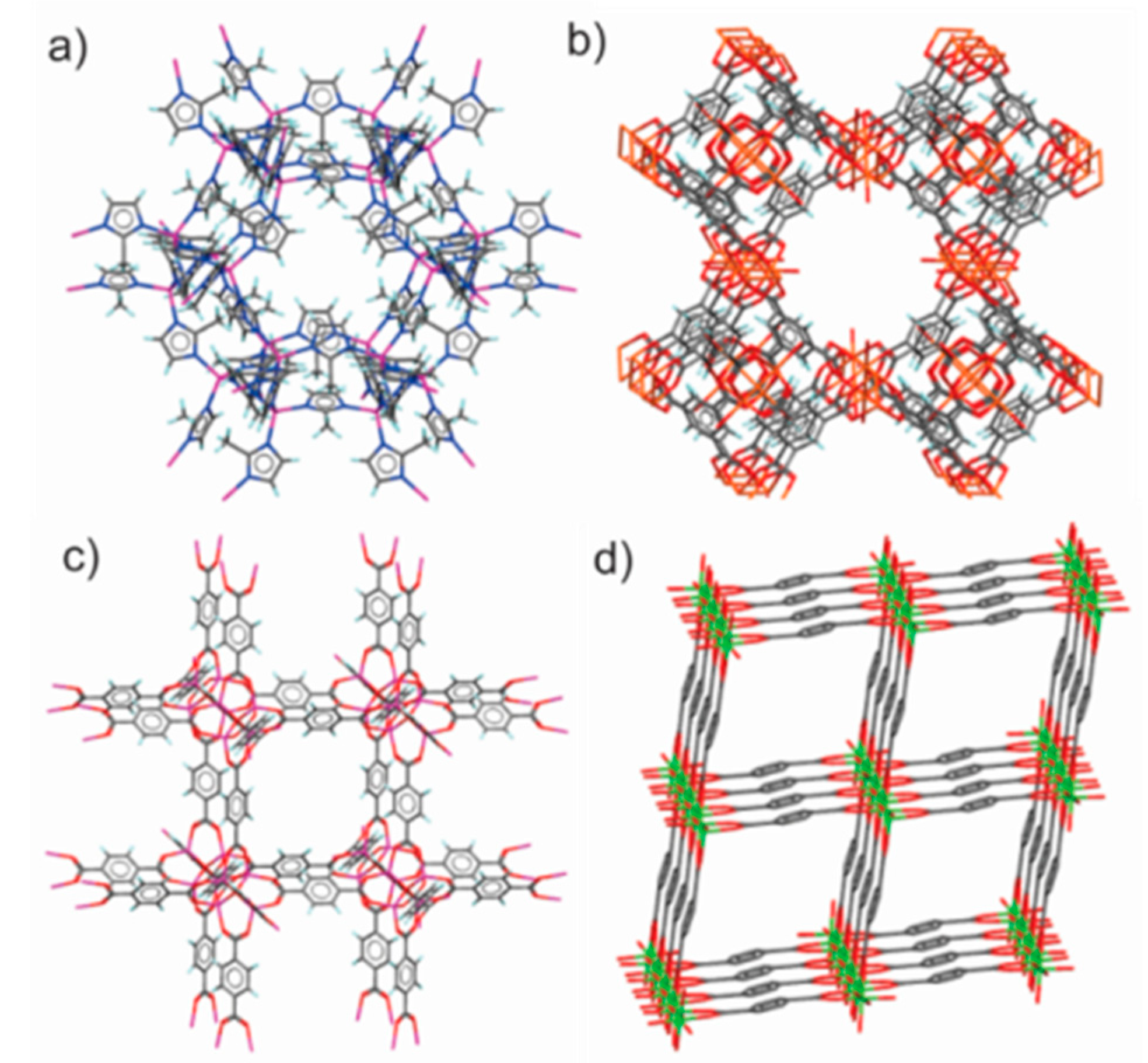
3.5. Mechanochemical Synthesis of MOFs by Neat Grinding
3.6. Mechanochemical Amorphization of MOFs
3.7. Mechanochemical Synthesis of MOFs Using LAG and ILAG Methodology
4. Recent Mechanistic Studies
4.1. Ex Situ (Stepwise) Monitoring of Mechanochemical Reactions
4.2. In Situ and Real-Time Monitoring of MOF Synthesis
5. Synthesis Assisted by Solvent Vapour
5.1. Vapour Digestion
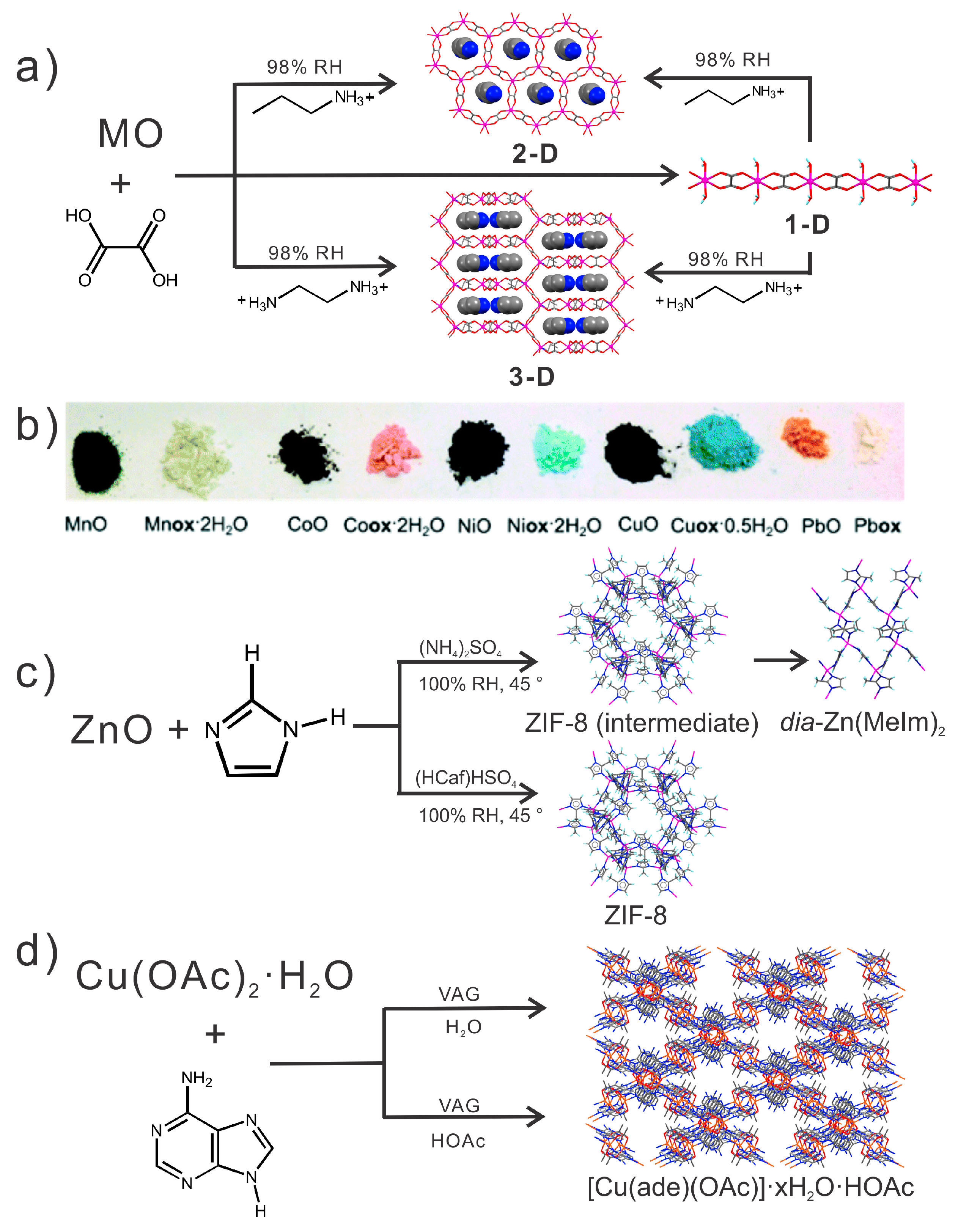
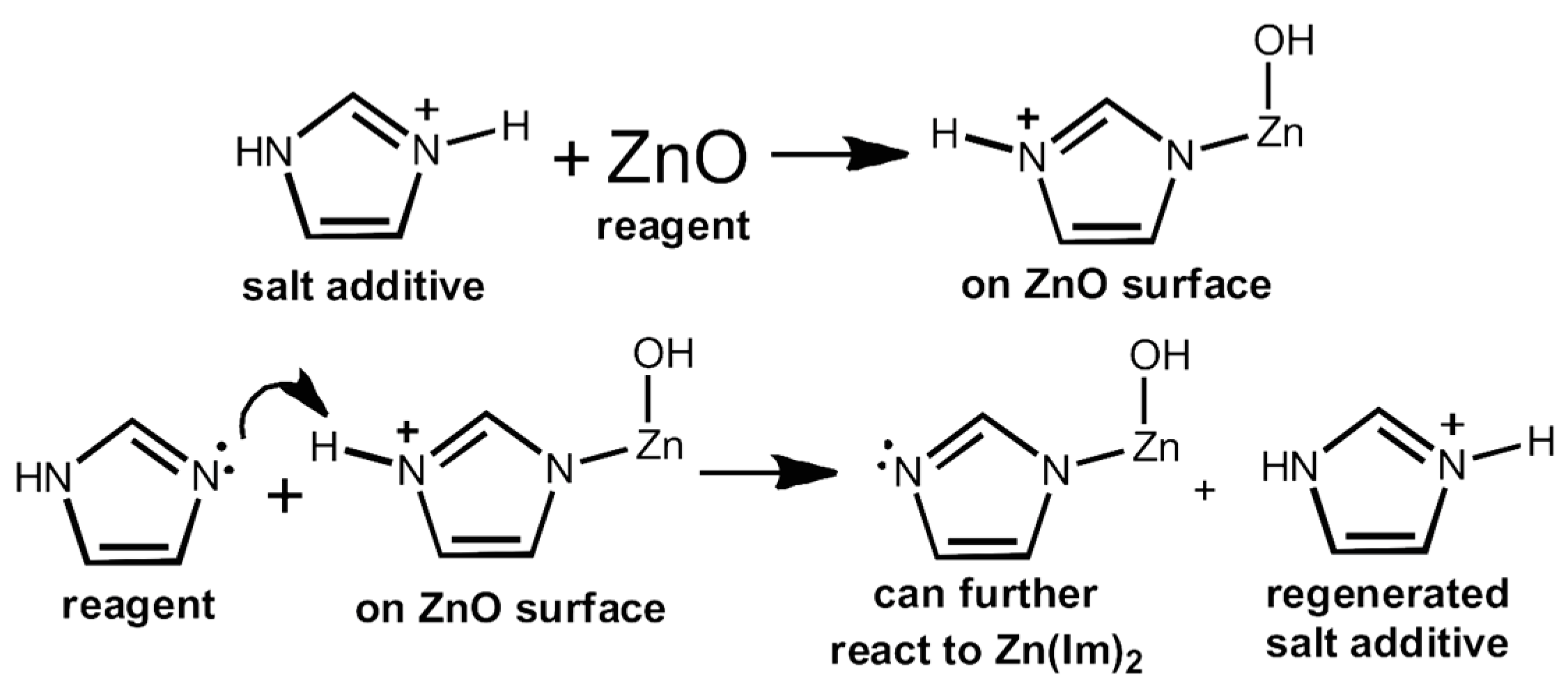
5.2. Accelerated Aging: Synthesis of MOFs Inspired by Mineral Neogenesis
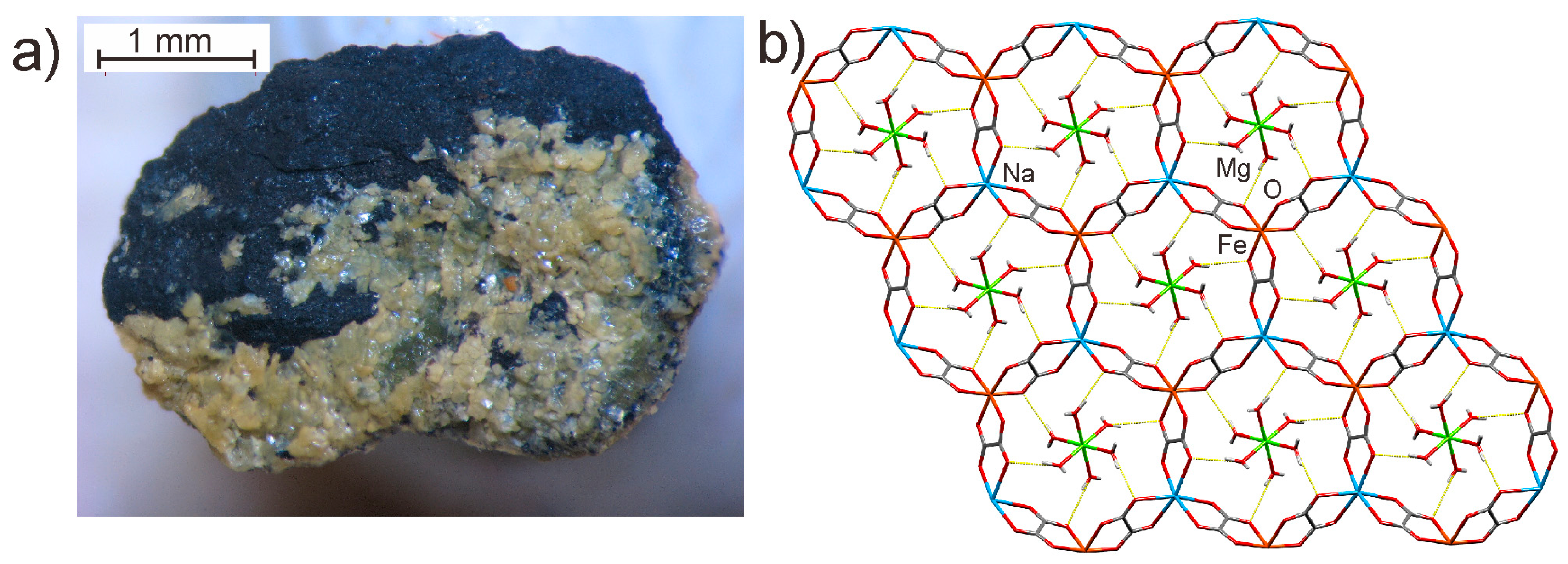
5.3. Geological Significance
5.4. Accelerated Aging as a Materials Testing Procedure
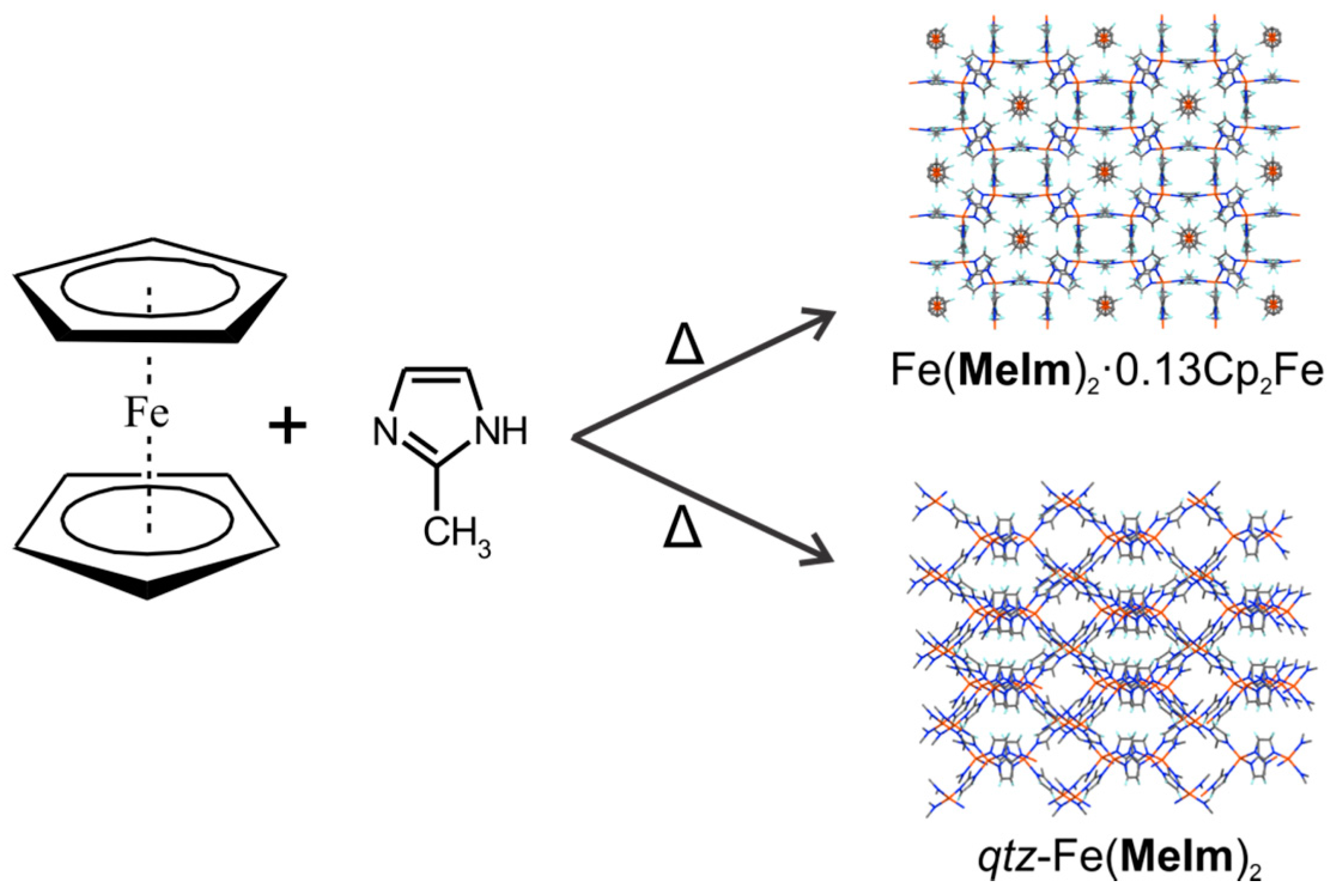
6. Thermochemical Solid-State Synthesis
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garay, A.L.; Pichon, A.; James, S.L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007, 36, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Giaffreda, S.L.; Grepioni, F.; Pettersen, A.; Maini, L.; Curzi, M.; Polito, M. Mechanochemical preparation of molecular and supramolecular organometallic materials and coordination networks. Dalton Trans. 2006, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Šepelák, V.; Bégin-Colin, S.; le Caër, G. Transformations in oxides induced by high-energy ball-milling. Dalton Trans. 2012, 41, 11927–11948. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, B.; Bruckmann, A.; Rantanen, T.; Bolm, C. Solvent-free carbon-carbon bond formations in ball mills. Adv. Synth. Catal. 2007, 349, 2213–2233. [Google Scholar] [CrossRef]
- Stolle, A.; Ondruschka, B. Solvent-free reactions of alkynes in ball mills: It is definitely more than mixing. Pure Appl. Chem. 2011, 83, 1343–1349. [Google Scholar] [CrossRef]
- Hernández, J.G.; Friščić, T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015, 56, 4253–4265. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Frameworks for commercial success (Editorial). Nat. Chem. 2016, 8, 987.
- Faust, T. MOFs move to market. Nat. Chem. 2016, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- An, A.J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework. Nat. Commun. 2012, 3, 604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.H.-L.; Xu, Q. Porous metal-organic frameworks as platforms for functional applications. Chem. Commun. 2011, 47, 3351–3370. [Google Scholar] [CrossRef] [PubMed]
- Meek, D.S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Kitagawa, S. Metal-organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.L.; Matsuda, R.; Kitagawa, S. Functional Hybrid Porous Coordination Polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Noro, A.S.; Mizutani, J.; Hijikata, Y.; Matsuda, R.; Sato, H.; Kitagawa, S.; Sugimoto, K.; Inubushi, Y.; Kubo, K.; Nakamura, T. Porous coordination polymers with ubiquitous and biocompatible metals and a neutral bridging ligand. Nat. Commun. 2015, 6, 5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the Zn(CN)2 and Cd(CN)2 structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4′′,4′′′-tetracyanotetraphenylmethane]BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar]
- Furukawa, H.; Go, Y.B.; Ko, N.; Park, Y.K.; Uribe-Romo, F.J.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Isoreticular Expansion of Metal-Organic Frameworks with Triangular and Square Building Units and the Lowest Calculated Density for Porous Crystals. Inorg. Chem. 2011, 50, 9147–9152. [Google Scholar] [CrossRef] [PubMed]
- Grünker, R.; Bon, V.; Müller, P.; Stoeck, U.; Krause, S.; Mueller, U.; Senkovska, I.; Kaskel, S. A new metal-organic framework with ultra-high surface area. Chem. Commun. 2014, 50, 3450–3452. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.Ö.; Hupp, J.T. Metal-Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Cavka, A.J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, B.V.; Ragon, F.; Dan-Hardi, M.; Devic, T.; Vishnuvarthan, M.; Campo, B.; Vimont, A.; Clet, G.; Yang, Q.; Maurin, G.; et al. A Series of Isoreticular, Highly Stable, Porous Zirconium Oxide Based Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 9267–9271. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Doonan, C.J.; Yaghi, O.M. Postsynthetic Modification of a Metal-Organic Framework for Stabilization of a Hemiaminal and Ammonia Uptake. Inorg. Chem. 2011, 50, 6853–6855. [Google Scholar] [CrossRef] [PubMed]
- Karagiaridi, B.O.; Vermeulen, N.A.; Klet, R.C.; Wang, T.C.; Moghadam, P.Z.; Al-Juaid, S.S.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. Functionalized Defects through Solvent-Assisted Linker Exchange: Synthesis, Characterization, and Partial Postsynthesis Elaboration of a Metal-Organic Framework Containing Free Carboxylic Acid Moieties. Inorg. Chem. 2015, 54, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Doonan, C.J.; Furukawa, H.; Banerjee, R.; Yaghi, O.M. Crystals as molecules: Postsynthesis covalent functionalization of zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2008, 130, 12626–12627. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-Y.; Liu, Y.; Hupp, J.T.; Farha, O.K. Instantaneous Hydrolysis of Nerve-Agent Simulants with a Six-Connected Zirconium-Based Metal-Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 6795–6799. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Gandára, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High Methane Storage Capacity in Aluminum Metal-Organic Frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, A.M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, B.V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A supermolecular building approach for the design and construction of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed]
- Nouar, C.F.; Eubank, J.F.; Bousquet, T.; Wojtas, L.; Zaworotko, M.J.; Eddaoudi, M. Supermolecular building blocks (SBBs) for the design and synthesis of highly porous metal-organic frameworks. J. Am. Chem. Soc. 2008, 130, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Latroche, M.; Serre, C.; Millange, F.; Loiseau, T.; Percheron-Guégan, A. Hydrogen adsorption in the nanoporous metal-benzenedicarboxylate M(OH)(O2C–C6H4–CO2)(M=Al3+, Cr3+), MIL-53. Chem. Commun. 2003, 2976–2977. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal-Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Xamena, L.I.X.F.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Müller, U.; Yaghi, O.M. “Heterogeneity within Order” in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 3417–3430. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Fundamentals of Green Chemistry; efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Proc. Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- James, S.L.; Friščić, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494–7496. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; James, S.L.; Boldyreva, E.V.; Bolm, C.; Jones, W.; Mack, J.; Steed, J.W.; Suslick, K. Highlights from Faraday discussion 170: Challenges and opportunities of modern mechanochemistry, Montreal, Canada, 2014. Chem. Commun. 2015, 51, 6248. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, G.A. Solvent-assisted mechanochemistry. Chem. Commun. 2013, 49, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, M.J.; Mottillo, C.; Stein, R.S.; Bučar, D.-K.; Friščić, T. Accelerated aging: A low energy, solvent-free alternative to solvothermal and mechanochemical synthesis of metal-organic materials. Chem. Sci. 2012, 3, 2495–2500. [Google Scholar] [CrossRef]
- Mottillo, C.; Lu, Y.; Pham, M.-H.; Cliffe, M.J.; Do, T.-O.; Friščić, T. Mineral neogenesis as an inspiration for mild, solvent-free synthesis of bulk microporous metal-organic frameworks from metal (Zn, Co) oxides. Green Chem. 2013, 15, 2121–2131. [Google Scholar] [CrossRef]
- Wen, L.-R.; Li, Z.-R.; Li, M.; Cao, H. Solvent-free and efficient synthesis of imidazo[1,2-a]pyridine derivatives via a one-pot three-component reaction. Green Chem. 2012, 14, 707–716. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zaworotko, M.J. Template-directed synthesis of metal-organic materials. Chem. Soc. Rev. 2014, 43, 5444–5455. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.D.C.; Clark, T.D.; Mack, J. Conducting moisture sensitive reactions under mechanochemical conditions. Tetrahedron Lett. 2012, 53, 4510–4513. [Google Scholar] [CrossRef]
- Tan, D.; Mottillo, C.; Katsenis, A.D.; Štrukil, V.; Friščić, T. Development of C-N Coupling Using Mechanochemistry: Catalytic Coupling of Arylsulfonamides and Carbodiimides. Angew. Chem. 2014, 126, 9321–9324. [Google Scholar] [CrossRef] [PubMed]
- Düvel, A.; Bednarcik, J.; Šepelák, V.; Heitjans, P. Mechanosynthesis of the Fast Fluoride Ion Conductor Ba1-xLaxF2+x: From the Fluorite to the Tysonite Structure. J. Phys. Chem. C 2014, 118, 7117–7129. [Google Scholar] [CrossRef]
- Hernández, J.G.; Butler, I.S.; Friščić, T. Multi-step and multi-component organometallic synthesis in one pot using orthogonal mechanochemical reactions. Chem. Sci. 2014, 5, 3576–3582. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P.; Rheingold, A.L. Mechanochemical Synthesis of [1,3-(SiMe3)2C3H3]3(Al,Sc), a Base-Free Tris(allyl)aluminum Complex and Its Scandium Analogue. Organometallics 2014, 33, 5952–5955. [Google Scholar] [CrossRef]
- Tan, A.D.; Štrukil, V.; Mottillo, C.; Friščić, T. Mechanosynthesis of pharmaceutically relevant sulfonyl-(thio)ureas. Chem. Commun. 2014, 50, 5248–5250. [Google Scholar] [CrossRef] [PubMed]
- Konnert, L.; Reneaud, B.; de Figueiredo, R.M.; Campagne, J.-M.; Lamaty, F.; Martinez, J.; Colacino, E. Mechanochemical Preparation of Hydantoins from Amino Esters: Application to the Synthesis of the Antiepileptic Drug Phenytoin. J. Org. Chem. 2014, 79, 10132–10142. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.; Balu, A.M.; Campelo, J.M.; Romero, A.A.; Carmona, D.; Balas, F.; Santamaria, J.; Luque, R. A Dry Milling Approach for the Synthesis of Highly Active Nanoparticles Supported on Porous Materials. ChemSusChem 2011, 4, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.J.; Saadé, N.K.; Friščić, T.; Moores, A. Mechanosynthesis of ultra-small monodisperse amine-stabilized gold nanoparticles with controllable size. Green Chem. 2014, 16, 86–89. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Yépez, A.; Luque, R.; Camacho, L.; de Miguel, G. Benign-by-Design Solventless Mechanochemical Synthesis of Three-, Two-, and One-Dimensional Hybrid Perovskites. Angew. Chem. Int. Ed. 2016, 55, 14972–14977. [Google Scholar] [CrossRef] [PubMed]
- Rajput, L.; Banerjee, R. Mechanochemical Synthesis of Amide Functionalized Porous Organic Polymers. Cryst. Growth Des. 2014, 14, 2729–2732. [Google Scholar] [CrossRef]
- Yang, H.; Orefuwa, S.; Goudy, A. Study of mechanochemical synthesis in the formation of the metal-organic framework Cu3(BTC)2 for hydrogen storage. Microporous Mesoporous Mater. 2011, 143, 37–45. [Google Scholar] [CrossRef]
- Morris, R.E.; James, S.L. Solventless Synthesis of Zeolites. Angew. Chem. Int. Ed. 2013, 52, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Giri, N.; Huang, X.; Apperley, D.; James, S.L. One-pot two-step mechanochemical synthesis: ligand and complex preparation without isolating intermediates. Green Chem. 2014, 16, 1374–1382. [Google Scholar] [CrossRef]
- Antesberger, J.; Cave, G.W.V.; Ferrarelli, M.C.; Heaven, M.W.; Raston, C.L.; Atwood, J.L. Solvent-free, direct synthesis of supramolecular nano-capsules. Chem. Commun. 2005, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Içli, B.; Christinat, N.; Tönnemann, J.; Schüttler, C.; Scopelliti, R.; Severin, K. Synthesis of Molecular Nanostructures by Multicomponent Condensation Reactions in a Ball Mill. J. Am. Chem. Soc. 2009, 131, 3154–3155. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.-X.; Qiu, Y.-M.; Li, M.; Zheng, J.; Sun, R.W.-Y.; Zhan, S.-Z.; Ng, S.W.; Li, D. Metallophilicity-driven dynamic aggregation of a phosphorescent gold(I)-silver(I) cluster prepared by solution-based and mechanochemical approaches. J. Am. Chem. Soc. 2014, 136, 9532–9535. [Google Scholar] [CrossRef] [PubMed]
- Medishetty, R.; Tandiana, R.; Koh, L.L.; Vittal, J.J. Assembly of 3D Coordination Polymers from 2D Sheets by [2 + 2] Cycloaddition Reaction. Chem. Eur. J. 2014, 20, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Belcher, W.J.; Longstaff, C.A.; Neckenig, M.R.; Steed, J.W. Channel-containing 1D coordination polymers based on a linear dimetallic spacer. Chem. Commun. 2002, 1602–1603. [Google Scholar] [CrossRef]
- Aboutorabi, L.; Morsali, A. Structural transformations and solid-state reactivity involving nano lead(II) coordination polymers via thermal, mechanochemical and photochemical approaches. Coord. Chem. Rev. 2016, 310, 116–130. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-organic frameworks (MOFs): Synthesis, structure and function. Acta Cryst. Sec. B. 2014, B70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.; Casaban, J.; Haydon, R.; Giri, N.; McNally, T.; James, S.L. Synthesis by extrusion: Continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 2015, 6, 1645–1649. [Google Scholar] [CrossRef]
- Crawford, D.E.; Casaban, J. Recent Developments in Mechanochemical Materials Synthesis by Extrusion. Adv. Mater. 2016, 28, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Lobo, R.F. Zeolite and molecular-sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Natarajan, S.; Cheetham, A.K.; Rao, C.N.R. New open-framework zinc oxalates synthesized in the presence of structure-directing organic amines. Chem. Mater. 1999, 11, 3636–3642. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Trask, A.V.; Shan, N.; Motherwell, W.D.S.; Jones, W.; Feng, S.; Tan, R.B.H.; Carpenter, K.J. Selective polymorph transformation via solvent-drop grinding. Chem. Commun. 2005, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; di Nicola, C.; Pettinari, C.; Skelton, B.W.; Somers, N.; White, A.H. Mechanochemical synthesis in copper(II) halide/pyridine systems: Single crystal X-ray diffraction and IR spectroscopic studies. Dalton Trans. 2011, 40, 5102–5115. [Google Scholar] [CrossRef] [PubMed]
- Kulla, H.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. In Situ Investigation of a Self-Accelerated Cocrystal Formation by Grinding Pyrazinamide with Oxalic Acid. Molecules 2016, 21, 917. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, G.A.; Hanna, J.V.; Skelton, B.W.; White, A.H. Mechanochemical and solution synthesis, and crystal structures and IR and solid-state (CPMAS) NMR spectroscopy of some bis(triphenylphosphine) silver(I) mono- and di-hydrogencitrate systems. Dalton Trans. 2012, 41, 5409–5417. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.L.; Frišćić, T.; Day, G.M.; Gladden, L.F.; Jones, W. Terahertz time-domain spectroscopy and the quantitativemonitoring of mechanochemical cocrystal formation. Nat. Mater. 2007, 6, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Šepelák, V.; Heitjans, P.; Becker, K.D. Nanoscale spinel ferrites prepared by mechanochemical route. J. Therm. Anal. Calorim. 2007, 90, 93–97. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Meštrović, E.; Šamec, D.Š.; Kaitner, B.; Fábián, L. One-Pot Mechanosynthesis with Three Levels of Molecular Self-Assembly: Coordination Bonds, Hydrogen Bonds and Host-Guest Inclusion. Chem. Eur. J. 2009, 15, 12644–12652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, F.; Hughes, C.E.; Browne, D.L.; Peskett, T.R.; Harris, K.D.M. Discovery of New Metastable Polymorphs in a Family of Urea Co-Crystals by Solid-State Mechanochemistry. Cryst. Growth Des. 2015, 15, 2901–2907. [Google Scholar] [CrossRef]
- Štrukil, V.; Igrc, M.D.; Fábián, L.; Eckert-Maksić, M.; Childs, L.S.; Reid, D.G.; Duer, M.J.; Halasz, I.; Mottillo, C.; Friščić, T. A model for a solvent-free synthetic organic research laboratory: Click-mechanosynthesis and structural characterization of thioureas without bulk solvents. Green Chem. 2012, 14, 2462–2473. [Google Scholar] [CrossRef]
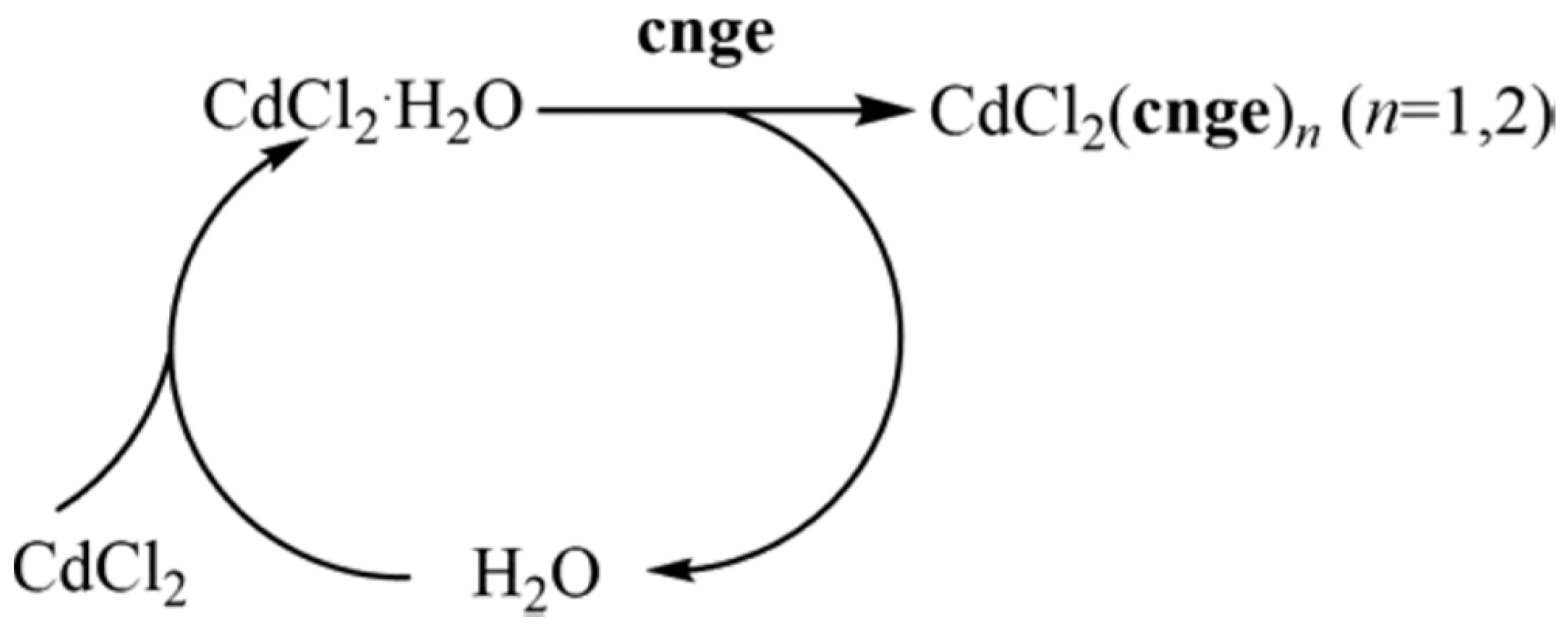
- Wilke, M.; Batzdorf, L.; Fischer, F.; Rademann, K.; Emmerling, F. Cadmium phenylphosphonates: Preparation, characterisation and in situ investigation. RSC Adv. 2016, 6, 36011–36019. [Google Scholar] [CrossRef]
- Klinowski, J.; Paz, F.A.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal-Organic Frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Pons-Balagué, A.; Ojea, M.J.H.; Ledezma-Gairaud, M.; Maneru, D.R.; Teat, S.J.; Costa, J.S.; Aromí, G.; Sanudo, E.C. Microwave assisted synthesis in coordination chemistry. Polyhedron 2013, 52, 781–787. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.; Honkimäki, V.; Dinnebier, R.E. Real-time and in situ monitoring of mechanochemical milling reactions. Nat. Chem. 2013, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Batzdorf, L.; Fischer, F.; Wilke, M.; Wenzel, K.-J.; Emmerling, F. Direct In Situ Investigation of Milling Reactions Using Combined X-ray Diffraction and Raman Spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Užarević, K.; Halasz, I.; Friščić, T. Real-Time and In Situ Monitoring of Mechanochemical Reactions: A New Playground for All Chemists. J. Phys. Chem. Lett. 2015, 6, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Jörres, M.; Acena, J.L.; Soloshonok, V.A.; Bolm, C. Asymmetric Carbon-Carbon Bond Formation under Solventless Conditions in Ball Mills. ChemCatChem. 2015, 7, 1265–1269. [Google Scholar] [CrossRef]
- Balestrin, L.B.D.; del Duque, D.; da Silva, D.S.; Galembeck, F. Triboelectricity in insulating polymers: Evidence for a mechanochemical mechanism. Faraday Discuss. 2014, 170, 369–383. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Chen, M.-W.; Dlott, D.D.; Suslick, K.S. Ultrasonic hammer produces hot spots in solids. Nat. Commun. 2015, 6, 6581. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.W.; Pill, M.F.; Kersch, A.; Clausen-Schaumann, H.; Beyer, M.K. Mechanically induced silyl ester cleavage under acidic conditions investigated by AFM-based single-molecule force spectroscopy in the force-ramp mode. Faraday Discuss. 2014, 170, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Takacs, L. Quicksilver from Cinnabar: The First Documented Mechanochemical Reaction? JOM 2000, 52, 12–13. [Google Scholar] [CrossRef]
- Lea, M.C. Transformations of Mechanical into Chemical Energy. (Third Paper.) Action of Shearing-Stress (Continued). Am. J. Sci. 1894, 47, 377–382. [Google Scholar] [CrossRef]
- Baláž, P.; Dutková, E. Fine milling in applied mechanochemistry. Miner. Eng. 2009, 22, 681–694. [Google Scholar] [CrossRef]
- Nachbaur, V.; Tauvel, G.; Verdier, T.; Jean, M.; Juraszek, J.; Houvet, D. Mecanosynthesis of partially inverted zinc ferrite. J. Alloys Compd. 2009, 473, 303–307. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Dutková, E.; Zorkovská, A.; Kováč, J.; Hronec, P.; Kováč, J., Jr.; Čaplovičová, M.; Mojžiš, j.; Mojžišová, G.; et al. CdS/ZnS nanocomposites: From mechanochemical synthesis to cytotoxicity issues. Mater. Sci. Eng. C 2016, 58, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, D.V.; Reva, V.P.; Kuryavy, V.G.; Petrov, V.V. Use of carbon compacts based on natural graphite for the mechanochemical synthesis of titanium carbide. Metallurgist 2012, 56, 456–461. [Google Scholar] [CrossRef]
- Rounaghi, S.A.; Eshghi, H.; Rashid, A.R.; Khaki, J.V.; Khoshkhoo, M.S.; Scudino, S.; Eckert, J. Synthesis of nanostructured AlN by solid state reaction of Al and diaminomaleonitrile. J. Solid State Chem. 2013, 198, 542–547. [Google Scholar] [CrossRef]
- Kim, J.W.; Shim, J.-H.; Ahn, J.-P.; Cho, Y.W.; Kim, J.-H.; Oh, K.H. Mechanochemical synthesis and characterization of TiB2 and VB2 nanopowders. Mater. Lett. 2008, 62, 2461–2464. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Aghili, S.E.; Enayati, M.H.; Karimzadeh, F. Synthesis of Nanocrystalline (Fe,Cr)3Al Powder by Mechanical Alloying. Mater. Manuf. Process. 2012, 27, 467–471. [Google Scholar] [CrossRef]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Patil, A.O.; Curtin, D.Y.; Paul, I.C. Formation of crystalline complexes between polymethylated quinones and hydroquinones. J. Chem. Soc. Perkin Trans. 1986, 2, 1687–1692. [Google Scholar] [CrossRef]
- Etter, M.C.; Reutzel, S.M.; Choo, C.G. Self-organization of adenine and thymine in the solid-state. J. Am. Chem. Soc. 1993, 115, 4411–4412. [Google Scholar] [CrossRef]
- Etter, M.C.; Siedle, A.R. Solid-state rearrangement of (phenylazophenyl)palladium hexafluoroacetate. J. Am. Chem. Soc. 1983, 105, 641–643. [Google Scholar] [CrossRef]
- Descamps, M.; Aumelas, A.; Desprez, S.; Willart, J.F. The amorphous state of pharmaceuticals obtained or transformed by milling: Sub-Tg features and rejuvenation. J. Non-Cryst. Solids 2015, 407, 72–80. [Google Scholar] [CrossRef]
- Hasa, D.; Rauber, G.S.; Voinovich, D.; Jones, W. Cocrystal Formation through Mechanochemistry: From Neat and Liquid-Assisted Grinding to Polymer-Assisted Grinding. Angew. Chem. Int. Ed. 2015, 54, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- Trask, A.V.; Haynes, D.A.; Motherwell, W.D.S.; Jones, W. Screening for crystalline salts via mechanochemistry. Chem. Commun. 2006, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Grepioni, F.; Maini, L.; Mazzeo, P.P.; Ventura, B. Solid-state reactivity of copper(I) iodide: Luminescent 2D-coordination polymers of CuI with saturated bidentate nitrogen bases. New. J. Chem. 2011, 35, 339–344. [Google Scholar] [CrossRef]
- Braga, D.; Giaffreda, S.L.; Grepioni, F.; Polito, M. Mechanochemical and solution preparation of the coordination polymers Ag[N(CH2CH2)3N]2[CH3COO]·5H2O and Zn[N(CH2CH2)3N]Cl2. CrystEngComm 2004, 6, 458–462. [Google Scholar] [CrossRef]
- Maini, L.; Braga, D.; Mazzeo, P.P.; Ventura, B. Polymorph and isomer conversion of complexes based on CuI and PPh3 easily observed via luminescence. Dalton Trans. 2012, 41, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Nagarathinam, M.; Chanthapally, A.; Lapidus, S.H.; Stephens, P.W.; Vittal, J.J. Mechanochemical reactions of coordination polymers by grinding with KBr. Chem. Commun. 2012, 48, 2585–2587. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Haddow, M.F.; Lusi, M.; Orpen, A.G. Crystal engineering of lattice metrics of perhalometallate salts and MOFs. Proc. Natl. Acad. Sci. USA 2010, 107, 16033–16038. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, L.; Morsali, A. Solid state structural transformation of 1D to intermediate 2D and then to 3D porous coordination polymer by anion replacement; new precursors for preparation of PbCl2, Pb3O2Cl2 and PbO nanoparticles. CrystEngComm 2013, 15, 8894–8897. [Google Scholar] [CrossRef]
- Grifasi, F.; Chierotti, M.R.; Garino, C.; Gobetto, R.; Priola, E.; Diana, E.; Turci, F. Solvent-Free Synthesis of Luminescent Copper(I) Coordination Polymers with Thiourea Derivatives. Cryst. Growth Des. 2015, 15, 2929–2939. [Google Scholar] [CrossRef]
- Chen, A.L.; Bovee, M.O.; Lemma, B.E.; Keithly, K.S.M.; Pilson, S.L.; Coleman, M.G.; Mack, J. An Inexpensive and Recyclable Silver-Foil Catalyst for the Cyclopropanation of Alkenes with Diazoacetates under Mechanochemical Conditions. Angew. Chem. Int. Ed. 2015, 54, 11084–11087. [Google Scholar] [CrossRef] [PubMed]
- Machuca, B.E.; Rojas, Y.; Juaristi, E. Synthesis and Evaluation of (S)-Proline-Containing α,β-Dipeptides as Organocatalysts in Solvent-Free Asymmetric Aldol Reactions Under Ball-Milling Conditions. Asian. J. Org. Chem. 2015, 4, 46–53. [Google Scholar] [CrossRef]
- Hermann, G.N.; Becker, P.; Bolm, C. Mechanochemical Rhodium(III)-Catalyzed C-H Bond Functionalization of Acetanilides under Solventless Conditions in a Ball Mill. Angew. Chem. Int. Ed. 2015, 54, 7414–7417. [Google Scholar] [CrossRef] [PubMed]
- Egbert, A.J.D.; Slawin, A.M.Z.; Nolan, S.P. Synthesis of N-Heterocyclic Carbene Gold Complexes Using Solution Phase and Solid-State Protocols. Organometallics 2013, 32, 2271–2274. [Google Scholar] [CrossRef]
- Hernández, J.G.; Bolm, C. [Cp*RhCl2]2: Mechanosynthesis and applications in C-H bond functionalisations under ball-milling conditions. Chem. Commun. 2015, 51, 12582–12584. [Google Scholar] [CrossRef] [PubMed]
- Rightmire, N.R.; Bruns, D.L.; Hanusa, T.P.; Brennessel, W.W. Mechanochemical Influence on the Stereoselectivity of Halide Metathesis: Synthesis of Group 15 Tris(allyl) Complexes. Organometallics 2016, 35, 1698–1706. [Google Scholar] [CrossRef]
- Toda, A.F.; Tanaka, K.; Sekikawa, A. Host-guest complex formation by a solid-solid reaction. J. Chem. Soc. Chem. Commun. 1987, 279–280. [Google Scholar] [CrossRef]
- Toda, F. Reaction control by a host guest complexation method. J. Incl. Phenom. Mol. Recognit. Chem. 1989, 7, 247–256. [Google Scholar] [CrossRef]
- Toda, F. Solid state organic chemistry: Efficient reactions, remarkable yields, and stereoselectivity. Acc. Chem. Res. 1995, 28, 480–486. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Štrukil, V.; Eckert-Maksić, M.; Dinnebier, R.E. Clean and Efficient Synthesis Using Mechanochemistry: Coordination Polymers, Metal-Organic Frameworks and Metallodrugs. Croat. Chem. Acta 2012, 85, 367–378. [Google Scholar] [CrossRef]
- Štrukil, V.; Bartolec, B.; Portada, T.; Ɖilović, I.; Halasz, I.; Margetić, D. One-pot mechanosynthesis of aromatic amides and dipeptides from carboxylic acids and amines. Chem. Commun. 2012, 48, 12100–12102. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Xu, K.; Clegg, J.K.; Ganguly, R.; Hirao, H.; Friščić, T.; Garcia, F. The first synthesis of the sterically encumbered adamantoid phosphazane P4((NtBu)6: Enabled by mechanochemistry. Angew. Chem. Int. Ed. 2016, 55, 12736–12740. [Google Scholar] [CrossRef] [PubMed]
- Štrukil, V.; Gracin, D.; Magdysyuk, O.V.; Dinnebier, R.E.; Friščić, T. Trapping Reactive Intermediates by Mechanochemistry: Elusive Aryl N-Thiocarbamoylbenzotriazoles as Bench-Stable Reagents. Angew. Chem. Int. Ed. 2015, 54, 8440–8443. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W.; Komatsu, K.; Murata, Y.; Shiro, M. Synthesis and x-ray structure of dumb-bell-shaped C120. Nature 1997, 387, 583–586. [Google Scholar]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Daurio, D.; Nagapudi, K.; Alvarez-Nunez, F. Manufacture of Pharmaceutical Co-Crystals Using Twin Screw Extrusion: A Solvent-Less and Scalable Process. J. Pharm. Sci. 2010, 99, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Daurio, D.; Nagapudi, K.; Li, L.; Quan, P.; Nunez, F.-A. Application of twin screw extrusion to the manufacture of cocrystals: Scale-up of AMG 517-sorbic acid cocrystal production. Faraday Discuss. 2014, 170, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Stojaković, J.; Farris, B.S.; MacGillivray, L.R. Vortex grinding for mechanochemistry: Application for automated supramolecular catalysis and preparation of a metal-organic framework. Chem. Commun. 2012, 48, 7958–7960. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Salamé, N.; Woo, S.; Bohle, D.S.; Friščić, T.; Cuccia, L.A. Rapid and facile solvent-free mechanosynthesis in a cell lysis mill: Preparation and mechanochemical complexation of aminobenzoquinones. CrystEngComm 2014, 16, 7180–7185. [Google Scholar] [CrossRef]
- Orita, A.; Jiang, L.; Nakano, T.; Ma, N.; Otera, J. Solventless reaction dramatically accelerates supramolecular self-assembly. Chem. Commun. 2002, 1362–1363. [Google Scholar] [CrossRef]
- Štrukil, V.; Fábián, L.; Reid, D.G.; Duer, M.J.; Jackson, G.J.; Eckert-Maksić, M.; Friščić, T. Towards an environmentally-friendly laboratory: Dimensionality and reactivity in the mechanosynthesis of metal-organic compounds. Chem. Commun. 2010, 46, 9191–9193. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Friščić, T.; Jones, W. Control and interconversion of cocrystal stoichiometry in grinding: Stepwise mechanism for the formation of a hydrogen-bonded cocrystal. CrystEngComm 2009, 11, 470–481. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. [Google Scholar] [CrossRef] [PubMed]
- Štrukil, V.; Igrc, M.D.; Eckert-Maksić, M.; Friščić, T. Click Mechanochemistry: Quantitative Synthesis of “Ready to Use” Chiral Organocatalysts by Efficient Two-Fold Thiourea Coupling to Vicinal Diamines. Chem. Eur. J. 2012, 18, 8464–8473. [Google Scholar] [CrossRef] [PubMed]
- Štrukil, V.; Margetić, D.; Igrc, M.D.; Eckert-Maksić, M.; Friščić, T. Desymmetrisation of aromatic diamines and synthesis of non-symmetrical thiourea derivatives by click-mechanochemistry. Chem. Commun. 2012, 48, 9705–9707. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, G.A.; Chaichit, N.; Pakawatchai, C.; Skelton, B.W.; White, A.H. Solvent-assisted mechanochemical synthesis of metal complexes. Dalton Trans. 2008, 2926–2928. [Google Scholar] [CrossRef] [PubMed]
- Thabet, S.K.; Tayim, H.A.; Karkanawi, M.U. Solid-phase synthetic inorganic reactions. Inorg. Nucl. Chem. Lett. 1972, 8, 211–213. [Google Scholar] [CrossRef]
- Bourne, S.A.; Kilkenny, M.; Nassimbeni, L.R. One- and two-dimensional coordination polymers of zinc(II) with pyrazine. Solid state reactions and decomposition kinetics of the interconversion reactions. J. Chem. Soc. Dalton Trans. 2001, 1176–1179. [Google Scholar] [CrossRef]
- Braga, D.; Curzi, M.; Grepioni, F.; Polito, M. Mechanochemical and solution reactions between AgCH3COO and [H2NC6H10NH2] yield three isomers of the coordination network {Ag[H2NC6H10NH2]+}∞. Chem. Commun. 2005, 2915–2917. [Google Scholar] [CrossRef] [PubMed]
- Akhbari, K.; Morsali, A. Structural conversion of cubane-type thallium complex to hexagon silver complex by solid-state mechanochemical reaction. Inorg. Chem. Commun. 2015, 55, 11–13. [Google Scholar] [CrossRef]
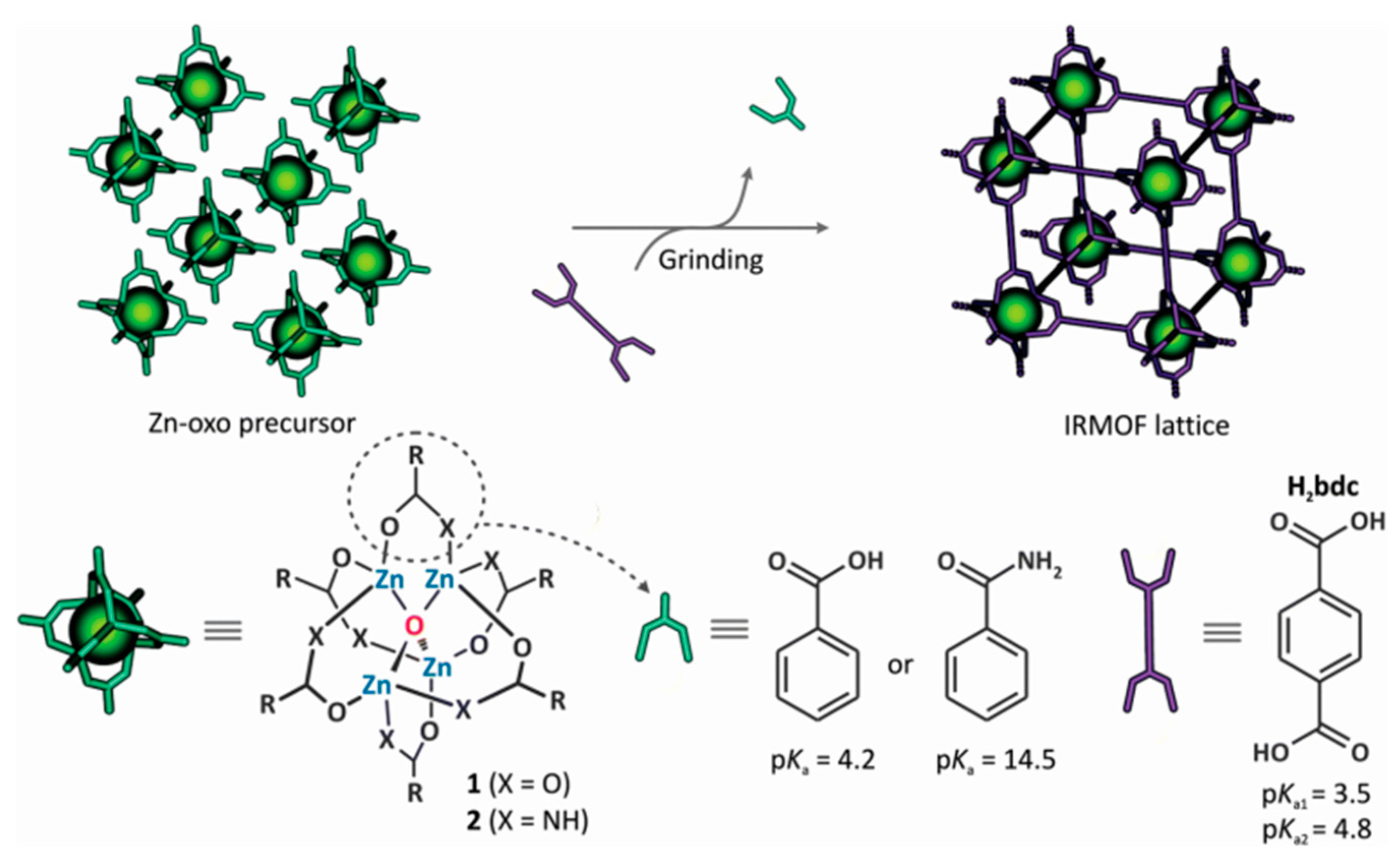
- Prochowicz, D.; Sokołowski, K.; Justyniak, I.; Kornowicz, A.; Fairen-Jimenez, D.; Friščić, T.; Lewiński, J. A mechanochemical strategy for IRMOF assembly based on pre-designed oxo-zinc precursors. Chem. Commun. 2015, 51, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Jobbágy, C.; Tunyogi, T.; Pálinkás, G.; Deák, A. A Versatile Solvent-Free Mechanochemical Route to the Synthesis of Heterometallic Dicyanoaurate-Based Coordination Polymers. Inorg. Chem. 2011, 50, 7301–7308. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Yoshida, J.; Nakamura, A.; Nishikiori, S. Annealing assisted mechanochemical syntheses of transition-metal coordination compounds and co-crystal formation. CrystEngComm 2009, 11, 427–432. [Google Scholar] [CrossRef]
- Yoshida, J.; Nishikiori, S.; Kuroda, R.; Yuge, H. Three Polymorphic CdII Coordination Polymers Obtained from the Solution and Mechanochemical Reactions of 3-Cyanopentane-2,4-dione with CdII Acetate. Chem. Eur. J. 2013, 19, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
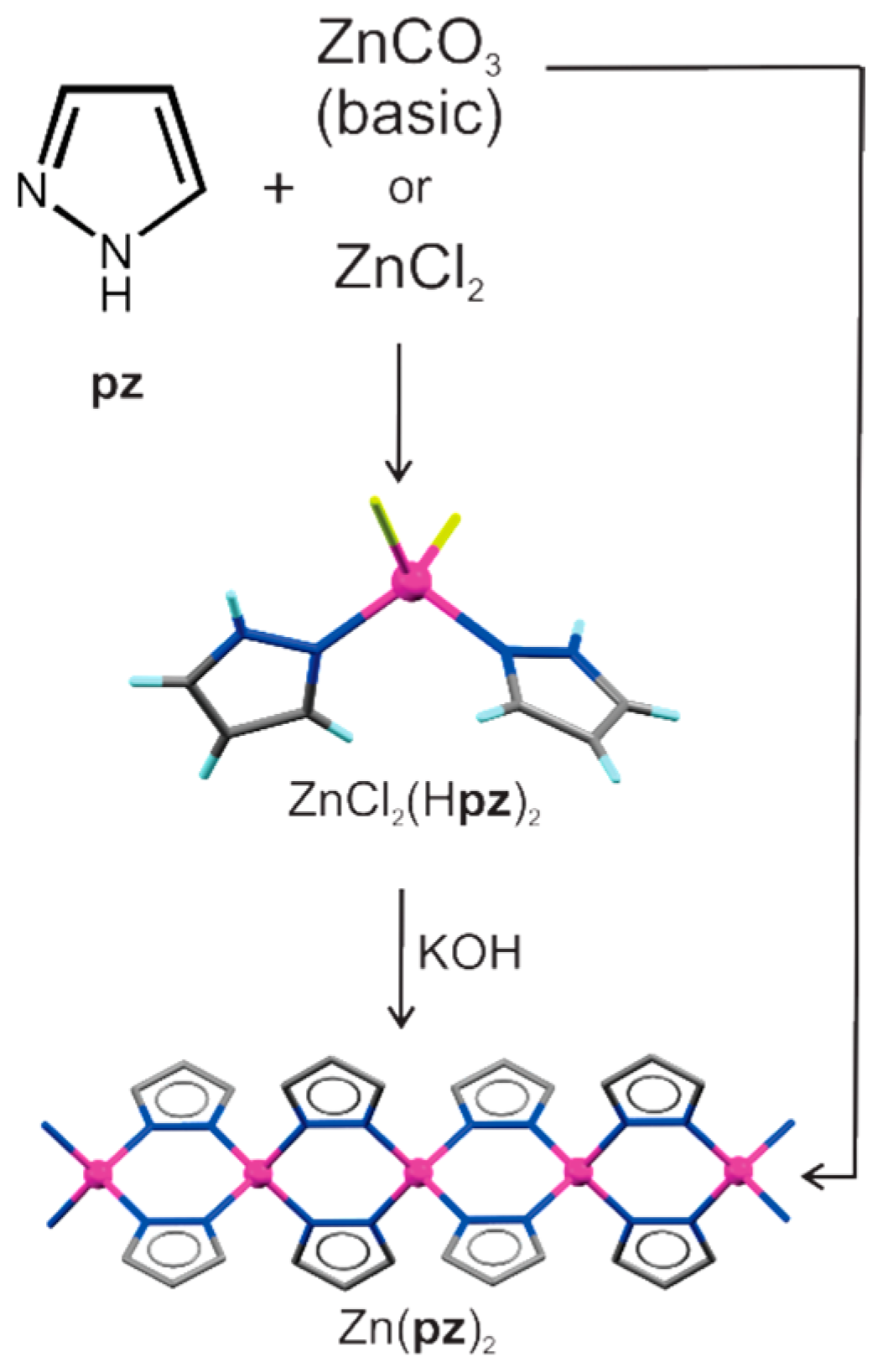
- Adams, C.J.; Kurawa, M.A.; Orpen, A.G. Coordination chemistry in the solid state: Synthesis and interconversion of pyrazolium salts, pyrazole complexes, and pyrazolate MOFs. Dalton Trans. 2010, 39, 6974–6984. [Google Scholar] [CrossRef] [PubMed]
- Michalchuk, A.A.L.; Tumanov, I.A.; Boldyreva, E.V. Complexities of mechanochemistry: Elucidation of processes occurring in mechanical activators via implementation of a simple organic system. CrystEngComm 2013, 15, 6403–6412. [Google Scholar] [CrossRef]
- Pichon, A.; James, S.L. An array-based study of reactivity under solvent-free mechanochemical conditions-insights and trends. CrystEngComm 2008, 10, 1839–1847. [Google Scholar] [CrossRef]
- Užarević, K.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Puškarić, A.; Friščić, T.; Halasz, I. Exploring the Effect of Temperature on a Mechanochemical Reaction by in Situ Synchrotron Powder X-ray Diffraction. Cryst. Growth Des. 2016, 16, 2342–2347. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Hasa, D.; Miniussi, E.; Jones, W. Mechanochemical Synthesis of Multicomponent Crystals: One Liquid for One Polymorph? A Myth to Dispel. Cryst. Growth Des. 2016, 16, 4582–4588. [Google Scholar] [CrossRef]
- Adams, C.J.; Colquhoun, H.M.; Crawford, P.C.; Lusi, M.; Orpen, A.G. Solid-state interconversions of coordination networks and hydrogen-bonded salts. Angew. Chem. Int. Ed. 2007, 46, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Curzi, M.; Johansson, A.; Polito, M.; Rubini, K.; Grepioni, F. Simple and quantitative mechanochemical preparation of a porous crystalline material based on a 1D coordination network for uptake of small molecules. Angew. Chem. Int. Ed. 2006, 45, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertran, J.F.; Hernández, M.P.; Reguera, E.; Yee-Madeira, H.; Rodriguez, J.; Paneque, A.; Llopiz, J.C. Characterization of mechanochemically synthesized imidazolates of Ag+1, Zn+2, Cd+2, and Hg+2: Solid state reactivity of nd10 cations. J. Phys. Chem. Solids 2006, 67, 1612–1617. [Google Scholar] [CrossRef]
- Friščić, T.; Fábián, L. Mechanochemical conversion of a metal oxide into coordination polymers and porous frameworks using liquid-assisted grinding (LAG). CrystEngComm 2009, 11, 743–745. [Google Scholar] [CrossRef]
- Fujii, K.; Garay, A.L.; Hill, J.; Sbircea, E.; Pan, Z.; Xu, M.; Apperley, D.C.; James, S.L.; Harris, K.D.M. Direct structure elucidation by powder X-ray diffraction of a metal-organic framework material prepared by solvent-free grinding. Chem. Commun. 2010, 46, 7572–7574. [Google Scholar] [CrossRef] [PubMed]
- Strobridge, F.C.; Judaš, N.; Friščić, T. A stepwise mechanism and the role of water in the liquid-assisted grinding synthesis of metal-organic materials. CrystEngComm 2010, 12, 2409–2418. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; André, V.; Duarte, T. Drug-containing coordination and hydrogen bonding networks obtained mechanochemically. CrystEngComm 2009, 11, 2618–2621. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Brescello, R.; Cotarca, L. Simple and quantitative mechanochemical preparation of the first zinc and copper complexes of the neuroleptic drug gabapentin. CrystEngComm 2008, 10, 469–471. [Google Scholar] [CrossRef]
- Quaresma, S.; André, V.; Antunes, A.M.M.; Cunha-Silva, L.; Duarte, M.T. Gabapentin Coordination Networks: Mechanochemical Synthesis and Behavior under Shelf Conditions. Cryst. Growth Des. 2013, 13, 5007–5017. [Google Scholar] [CrossRef]
- Domingos, S.; André, V.; Quaresma, S.; Martins, I.C.B.; da Piedade, M.F.M.; Duarte, M.T. New forms of old drugs: Improving without changing. J. Pharm. Pharmacol. 2015, 67, 830–846. [Google Scholar] [CrossRef] [PubMed]
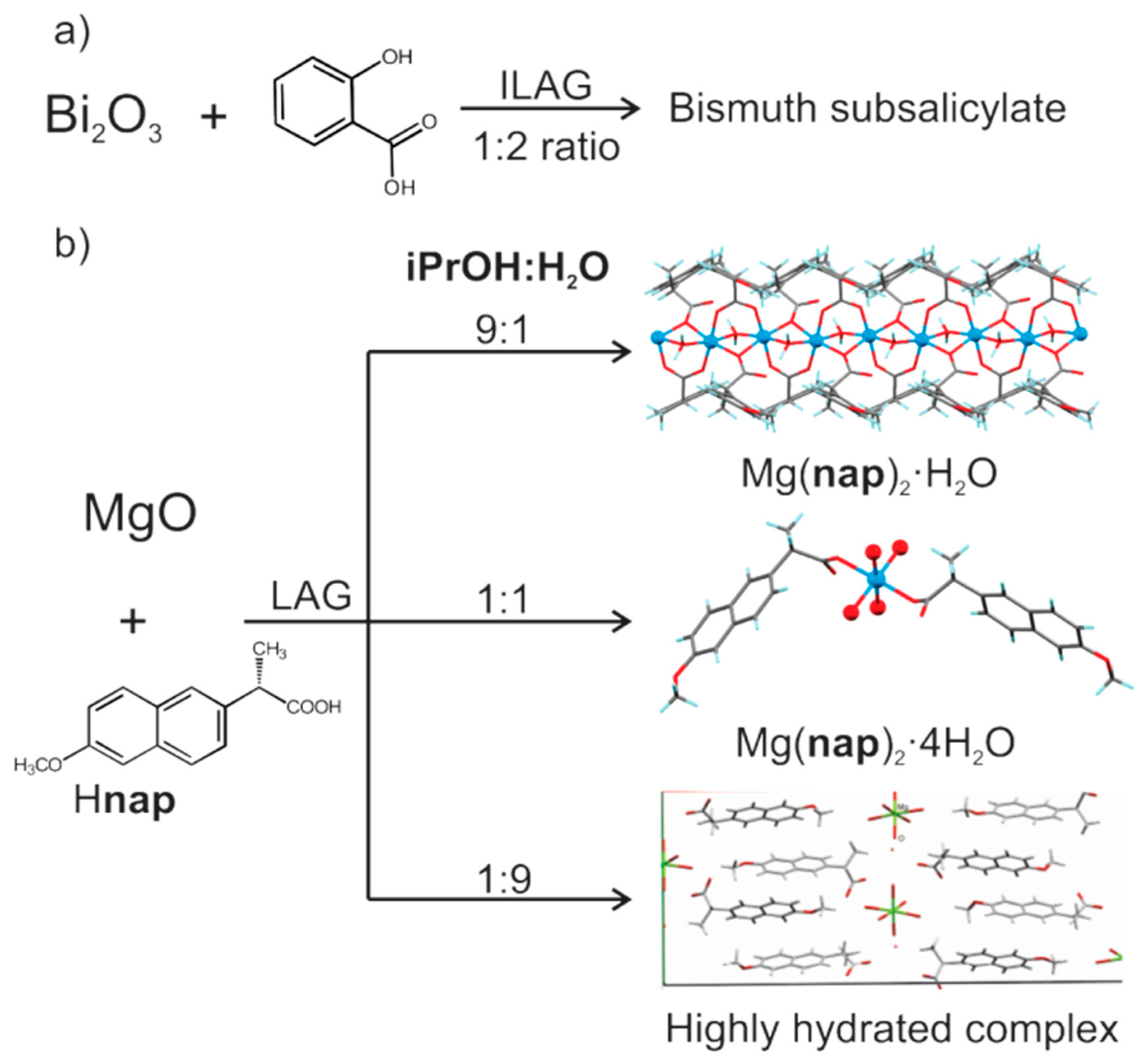
- André, V.; Hardeman, A.; Halasz, I.; Stein, R.S.; Jackson, G.J.; Reid, D.G.; Duer, M.J.; Curfs, C.; Duarte, M.T.; Friščić, T. Mechanosynthesis of the Metallodrug Bismuth Subsalicylate from Bi2O3 and Structure of Bismuth Salicylate without Auxiliary Organic Ligands. Angew. Chem. Int. Ed. 2011, 50, 7858–7861. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Deacon, G.B.; Junk, P.C.; Kumar, I.; Silberstein, M. Synthetic and structural comparisons of bismuth(III) carboxylates synthesised under solvent-free and reflux conditions. Dalton Trans. 2006, 4852–4858. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.H.H.; Strobridge, F.; Friščić, T. Mechanochemistry of magnesium oxide revisited: Facile derivatisation of pharmaceuticals using coordination and supramolecular chemistry. Chem. Commun. 2010, 46, 6368–6370. [Google Scholar] [CrossRef] [PubMed]
- Parojčić, J.; Corrigan, O.I. Rationale for ibuprofen co-administration with antacids: Potential interaction mechanisms affecting drug absorption. Eur. J. Pharm. Biopharm. 2008, 69, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Halasz, I.; Strobridge, F.C.; Dinnebier, R.E.; Stein, R.S.; Fábián, L.; Curfs, C. A rational approach to screen for hydrated forms of the pharmaceutical derivative magnesium naproxen using liquid-assisted grinding. CrystEngComm 2011, 13, 3125–3129. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous metal-organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
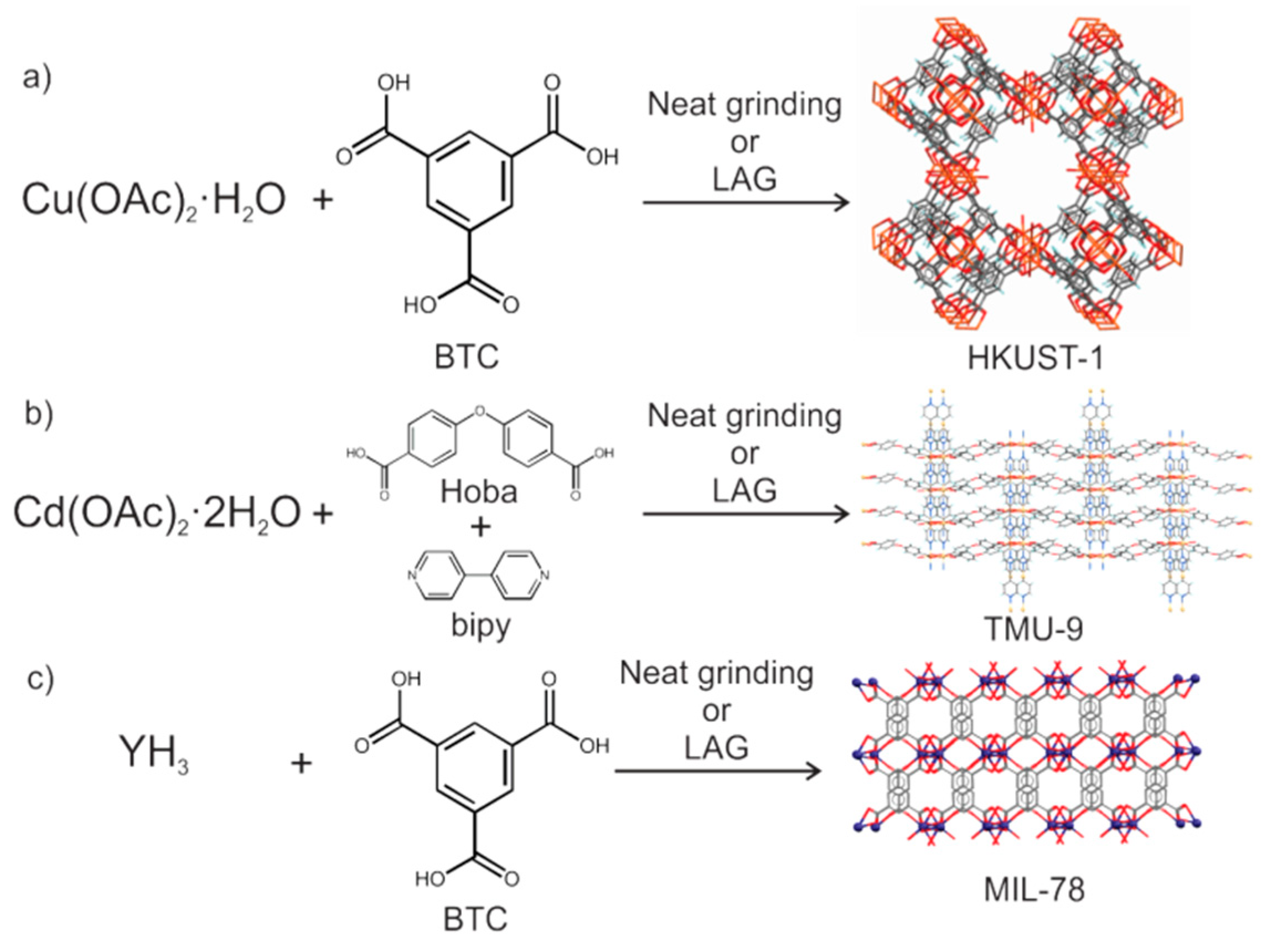
- Yuan, W.; Garay, A.L.; Pichon, A.; Clowes, R.; Wood, C.D.; Cooper, A.I.; James, S.L. Study of the mechanochemical formation and resulting properties of an archetypal MOF: Cu3(BTC)2 (BTC=1,3,5-benzenetricarboxylate). CrystEngComm 2010, 12, 4063–4065. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thünemann, F.; Rademann, K.; Emmerling, F. Mechanochemical Synthesis of Metal-Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Rademann, K.; Emmerling, F. Characterization of mechanochemically synthesized MOFs. Microporous Mesoporous Mater. 2012, 154, 113–118. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. Rapid mechanochemical synthesis of two new Cd(II)-based metal-organic frameworks with high removal efficiency of Congo red. CrystEngComm 2015, 17, 686–692. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Veith, G.M.; Dai, S. Soluble Porous Coordination Polymers by Mechanochemistry: From Metal-Containing Films/Membranes to Active Catalysts for Aerobic Oxidation. Adv. Mater. 2015, 27, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Hardi, M.; Balema, V.P. Mechanochemical synthesis of an yttrium based metal-organic framework. Chem. Commun. 2013, 49, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Trukhan, N.; Müller, U. Industrial Outlook on Zeolites and Metal Organic Frameworks. Chin. J. Catal. 2012, 33, 3–10. [Google Scholar] [CrossRef]
- Czaja, A.; Leung, E.; Trukhan, N.; Müller, U. Industrial MOF Synthesis. In Metal-Organic Frameworks: Applications from Catalysis to Gas Storage, 1st ed.; Farrusseng, D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Friščić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal-Organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem. Int. Ed. 2010, 49, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Friščić, T.; Apperley, D.; James, S.L. High Reactivity of Metal-Organic Frameworks under Grinding Conditions: Parallels with Organic Molecular Materials. Angew. Chem. Int. Ed. 2010, 49, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; O’Connor, J.; James, S.L. Mechanochemical synthesis of homo- and hetero-rare-earth(III) metal-organic frameworks by ball milling. CrystEngComm 2010, 12, 3515–3517. [Google Scholar] [CrossRef]
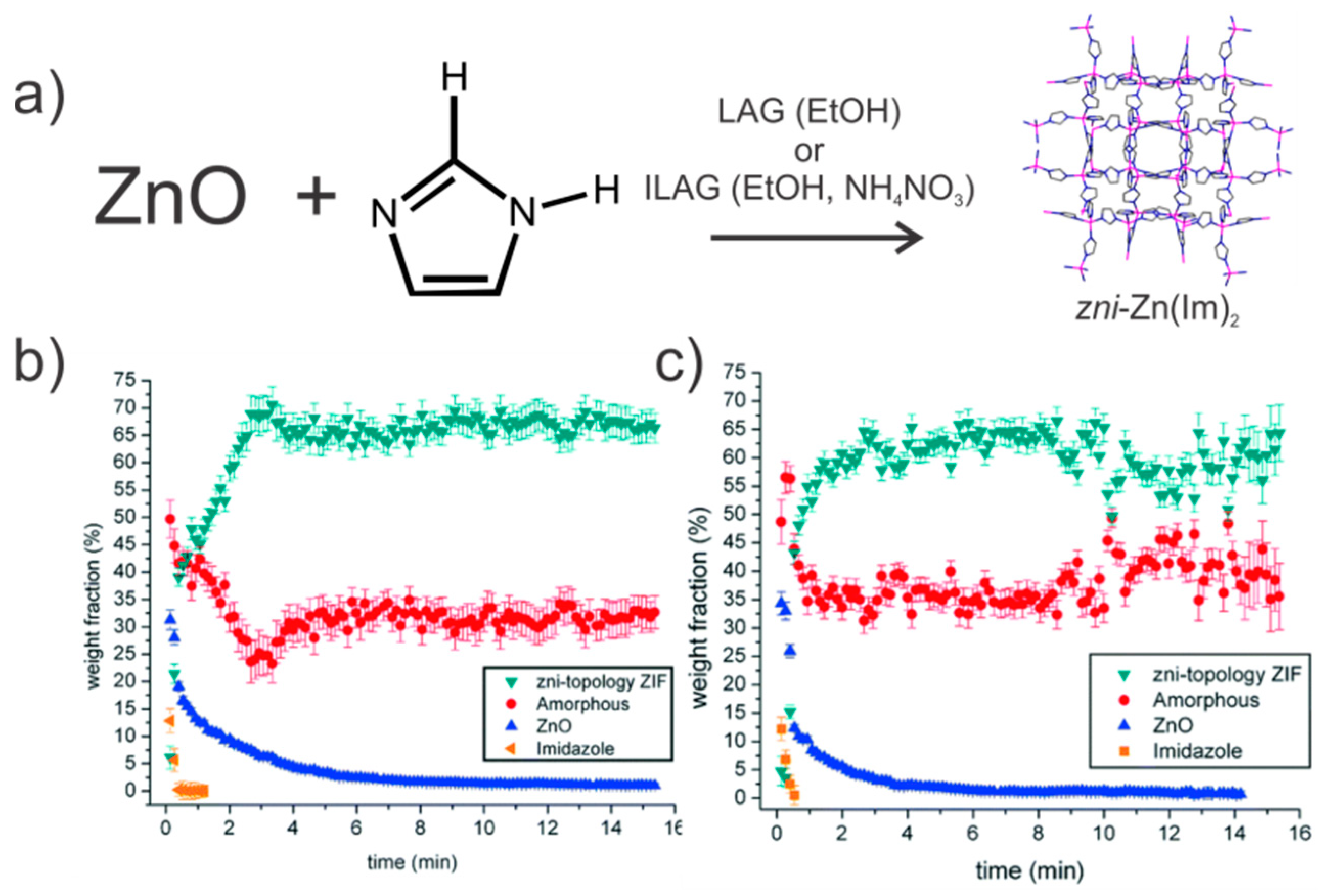
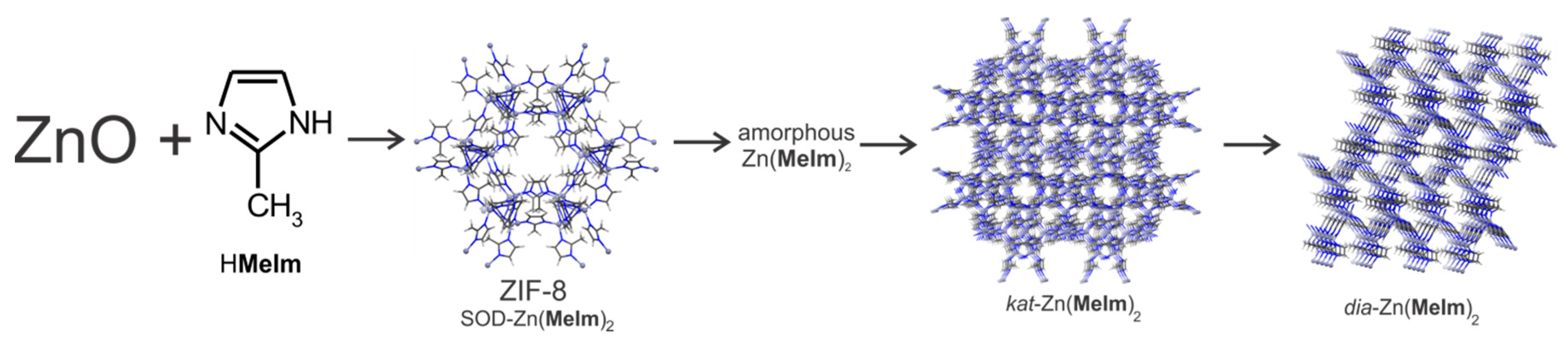
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks by Using Mechanochemistry. Angew. Chem. Int. Ed. 2010, 49, 9640–9643. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kida, K.; Nagaoka, T.; Ota, T.; Miyake, Y. Mechanochemical dry conversion of zinc oxide to zeolitic imidazolate framework. Chem. Commun. 2013, 49, 7884–7886. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Cheetham, A.K. Amorphous Metal-Organic Frameworks. Acc. Chem. Res. 2014, 47, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.F.; Bennett, T.D.; Cairns, A.B.; Brownbill, N.J.; Goodwin, A.L.; Keen, D.A.; Chater, P.A.; Blanc, F.; Cheetham, A.K. A comparison of the amorphization of zeolitic imidazolate frameworks (ZIFs) and aluminosilicate zeolites by ball-milling. Dalton Trans. 2016, 45, 4258–4268. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Cao, S.; Tan, J.C.; Keen, D.A.; Bithell, E.G.; Beldon, P.J.; Friscic, T.; Cheetham, A.K. Facile Mechanosynthesis of Amorphous Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2011, 133, 14546–14549. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Todorova, T.K.; Baxter, E.F.; Reid, D.G.; Gervais, C.; Bueken, B.; van de Voorde, B.; de Vos, D.; Keen, D.A.; Mellot-Draznieks, C. Connecting defects and amorphization in UiO-66 and MIL-140 metal-organic frameworks: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2016, 18, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Bennett, T.D.; Keen, D.A.; Goodwin, A.L.; Cheetham, A.K. Amorphization of the prototypical zeolitic imidazolate framework ZIF-8 by ball-milling. Chem. Commun. 2012, 48, 7805–7807. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.W.; Jelfs, K.E.; Konstas, K.; Doherty, C.M.; Hill, A.J.; Cheetham, A.K.; Bennett, T.D. Porosity in metal-organic framework glasses. Chem. Commun. 2016, 52, 3750–3753. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Kurawa, M.A.; Lusi, M.; Orpen, A.G. Solid state synthesis of coordination compounds from basic metal salts. CrystEngComm 2008, 10, 1790–1795. [Google Scholar] [CrossRef]
- Užarević, K.; Wang, T.C.; Moon, S.-Y.; Fidelli, A.M.; Hupp, J.T.; Farha, O.K.; Friščić, T. Mechanochemical and solvent-free assembly of zirconium-based metal-organic frameworks. Chem. Commun. 2016, 52, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Matoga, D.; Oszajca, M.; Molenda, M. Ground to conduct: Mechanochemical synthesis of a metal-organic framework with high proton conductivity. Chem. Commun. 2015, 51, 7637–7640. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Kurawa, M.A.; Orpen, A.G. Coordination Chemistry in the Solid State: Reactivity of Manganese and Cadmium Chlorides with Imidazole and Pyrazole and Their Hydrochlorides. Inorg. Chem. 2010, 49, 10475–10485. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2012, 14, 492–498. [Google Scholar] [CrossRef]
- Tumanov, I.A.; Achkasov, A.F.; Boldyreva, E.V.; Boldyrev, V.V. About the possibilities to detect intermediate stages in mechanochemical synthesis of molecular complexes. Russ. J. Phys. Chem. A 2012, 86, 1014–1017. [Google Scholar] [CrossRef]
- Lewis, D.W.; Ruiz-Salvador, A.R.; Gómez, A.; Rodriguez-Albelo, L.M.; Coudert, F.-X.; Slater, B.; Cheetham, A.K.; Mellot-Draznieks, C. Zeolitic imidazole frameworks: Structural and energetics trends compared with their zeolite analogues. CrystEngComm 2009, 11, 2272–2276. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, W.; Bell, S.E.J.; James, S.L. Better understanding of mechanochemical reactions: Raman monitoring reveals surprisingly simple ‘pseudo-fluid’ model for a ball milling reaction. Chem. Commun. 2014, 50, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Tröbs, L.; Emmerling, F. Mechanochemical synthesis and characterisation of cocrystals and metal organic compounds. Faraday Discuss. 2014, 170, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gracin, D.; Štrukil, V.; Friščić, T.; Halasz, I.; Užarević, K. Laboratory Real-Time and In Situ Monitoring of Mechanochemical Milling Reactions by Raman Spectroscopy. Angew. Chem. Int. Ed. 2014, 126, 6193–6197. [Google Scholar] [CrossRef] [PubMed]
- Halasz, I.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimäki, V.; Nightingale, R.C.; Dinnebier, R.E.; Friščić, T. In situ and real-time monitoring of mechanochemical milling reactions using synchrotron X-ray diffraction. Nat. Protoc. 2013, 8, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Halasz, I.; Friščić, T.; Kimber, S.A.J.; Užarević, K.; Puškarić, A.; Mottillo, C.; Julien, P.; Štrukil, V.; Honkimäki, V.; Dinnebier, R.E. Quantitative in situ and real-time monitoring of mechanochemical reactions. Faraday Discuss. 2014, 170, 203–221. [Google Scholar] [CrossRef] [PubMed]
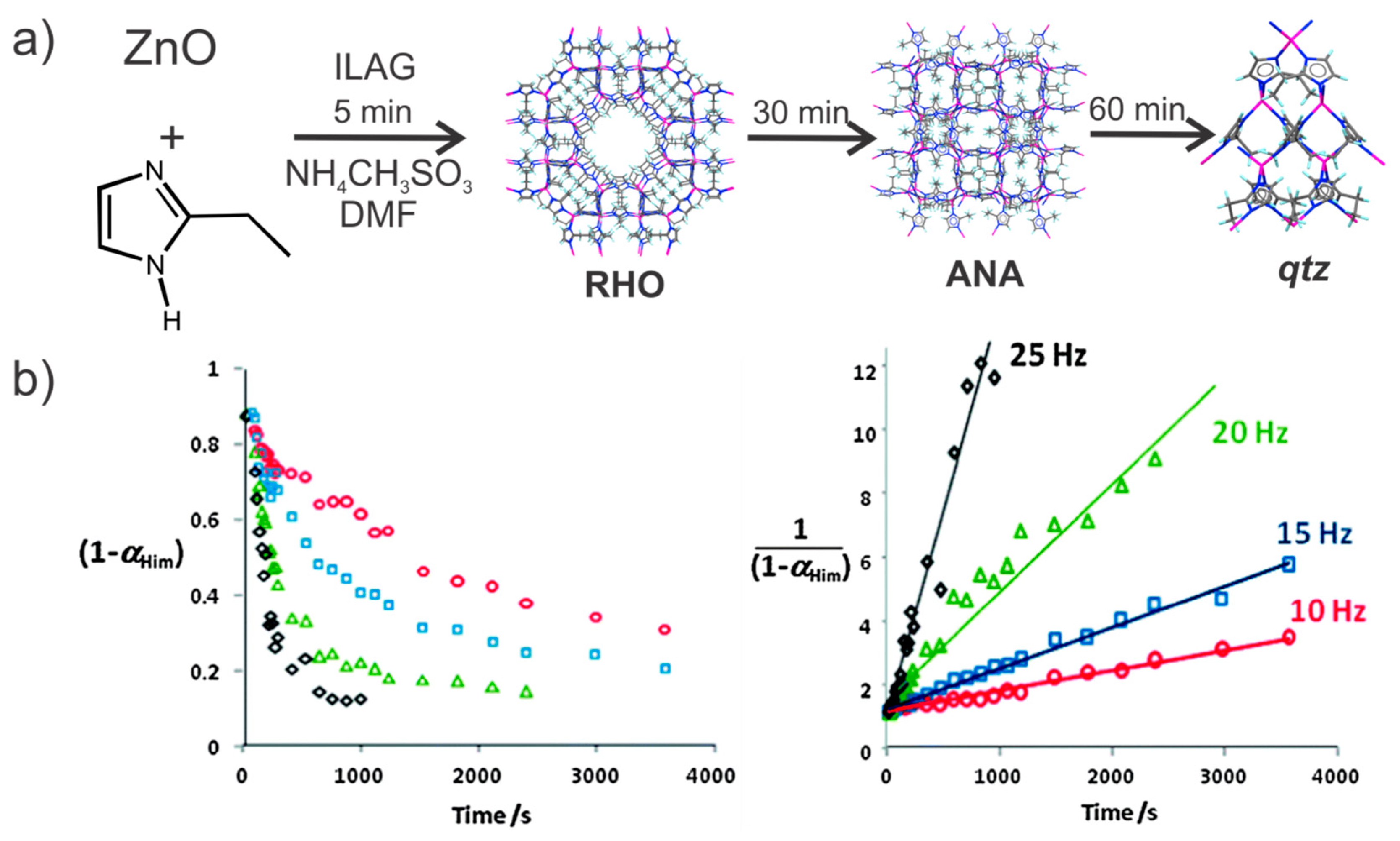
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.; Do, T.-O.; Kimber, S.A.J.; Lazić, P.; et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Stowell, J.G.; Morris, K.R.; Byrn, S.R. Quantitative study of solid-state acid-base reactions between polymorphs of flufenamic acid and magnesium oxide using X-ray powder diffraction. J. Pharm. Biomed. Anal. 2010, 51, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Byrn, S.R.; Xu, W.; Newman, A.W. Chemical reactivity in solid-state pharmaceuticals: Formulation implications. Adv. Drug Deliv. Rev. 2001, 48, 115–136. [Google Scholar] [CrossRef]
- Braga, D.; Giaffreda, S.L.; Grepioni, F.; Chierotti, M.R.; Gobetto, R.; Palladino, G.; Polito, M. Solvent effect in a “solvent free” reaction. CrystEngComm 2007, 9, 879–881. [Google Scholar] [CrossRef]
- Qi, F.; Stein, R.S.; Friščić, T. Mimicking mineral neogenesis for the clean synthesis of metal-organic materials from mineral feedstocks: Coordination polymers, MOFs and metal oxide separation. Green Chem. 2014, 16, 121–132. [Google Scholar] [CrossRef]
- Adamo, P.; Violante, P. Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl. Clay Sci. 2000, 16, 229–256. [Google Scholar] [CrossRef]
- Chen, J.; Blume, H.-P.; Beyer, L. Weathering of rocks induced by lichen colonization—A review. Catena 2000, 39, 121–146. [Google Scholar] [CrossRef]
- Jia, C.; Wang, J.; Feng, X.; Lin, Q.; Yuan, W. Efficient vapour-assisted aging and liquid-assisted grinding synthesis of a microporous copper-adeninate framework. CrystEngComm 2014, 16, 6552–6555. [Google Scholar] [CrossRef]
- Qi, F.; Stein, R.S.; Friščić, T. Separation of Metals in Mixture of Metallic Compounds Involves Mixing Mixture with Carboxylic Acid, Selectively Converting Metallic Compound(s) into Metal Carboxylate Complex and Separating Complex from Unreacted Metallic Compounds. U.S. 61/826,172, 22 May 2013. [Google Scholar]
- Feng, X.; Jia, C.; Wang, J.; Cao, X.; Tang, P.; Yuan, W. Efficient vapor-assisted aging synthesis of functional and highly crystalline MOFs from CuO and rare earth sesquioxides/carbonates. Green Chem. 2015, 17, 3740–3745. [Google Scholar] [CrossRef]
- Tang, P.; Jia, C.; Jiang, Y.; Gong, W.; Cao, X.; Yang, J.; Yuan, W. Reactivity Studies of Metal-Organic Frameworks under Vapor-Assisted Aging: Structural Interconversions and Transformations. Eur. J. Inorg. Chem. 2016, 5617–5622. [Google Scholar] [CrossRef]
- Huskić, I.; Pekov, I.V.; Krivovichev, S.V.; Friščić, T. Minerals with metal-organic framework structures. Sci. Adv. 2016, 2, e1600621. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Ensling, J.; Guetlich, P.A. A concept for the synthesis of 3-dimensional homometallic and bimetallic oxalate-bridged networks [M2(ox)3]n. Structural, Mössbauer, and magnetic studies in the field of molecular-based magnets. J. Am. Chem. Soc. 1994, 116, 9521–9528. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Yamada, T.; Honda, K.; Matsui, H.; Kitagawa, H. Control of Crystalline Proton-Conducting Pathways by Water-Induced Transformations of Hydrogen-Bonding Networks in a Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 7701–7707. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Nishikiori, S.; Kitazawa, T.; Yuge, H. Mineralomimetic chemistry as a modern aspect of co-ordination chemistry. Dalton Trans. 1997, 4127–4136. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Küsgens, P.; Rose, M.; Senkovska, I.; Frode, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal-organic frameworks by water adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
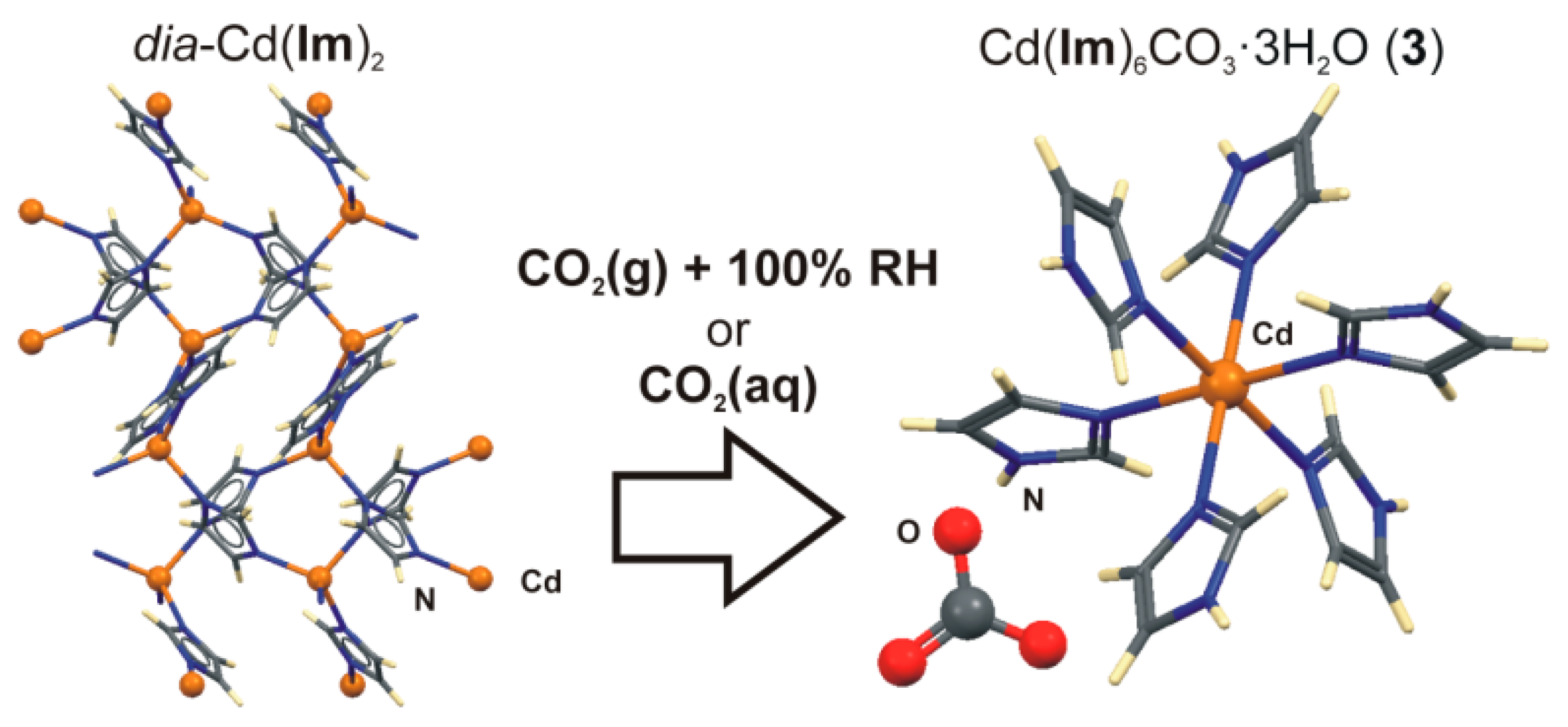
- Elsaidi, S.K.; Mohamed, M.H.; Schaef, H.T.; Kumar, A.; Lusi, M.; Pham, T.; Forrest, K.A.; Space, B.; Xu, W.; Halder, G.J.; et al. Hydrophobic pillared square grids for selective removal of CO2 from simulated flue gas. Chem. Commun. 2015, 51, 15530–15533. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, C.; Friščić, T. Carbon Dioxide Sensitivity of Zeolitic Imidazolate Frameworks. Angew. Chem. Int. Ed. 2014, 53, 7471–7474. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. Int. Ed. 2011, 50, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Matzger, A. Water Stability of Microporous Coordination Polymers and the Adsorption of Pharmaceuticals from Water. Langmuir 2010, 26, 17198–17202. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, S.A.; Su, J.; Zou, X.; Balkus, K.J., Jr. Carbonate-Based Zeolitic Imidazolate Framework for Highly Selective CO2 Capture. Inorg. Chem. 2015, 54, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Müller-Buschbaum, K.; Mokaddem, Y. MOFs by solvent free high temperature synthesis exemplified by ∞3[Eu3(Tz*)6(Tz*H)2]. Solid State Sci. 2008, 10, 416–420. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Schönfeld, F. The Utilisation of Solvent-Free Synthesis for the Reaction of Cobalt with Imidazole: MOF Conversion from ∞3[Co3(Im)6(ImH)2] via ∞3[Co4(Im)8(ImH)] to ∞3[Co(Im)2]. Z. Anorg. Allg. Chem. 2011, 637, 955–960. [Google Scholar] [CrossRef]
- Rettig, S.J.; Storr, A.; Summers, D.A.; Thompson, R.C.; Trotter, J. Iron(II) 2-methylimidazolate and copper(II) 1,2,4-triazolate complexes: Systems exhibiting long-range ferromagnetic ordering at low temperatures. Can. J. Chem. 1999, 77, 425–433. [Google Scholar] [CrossRef]
- Spek, A.L.; Duisenberg, A.J.M.; Feiters, M.C. The structure of the three-dimensional polymer poly[μ-hexakis(2-methylimidazolato-N,N')-triiron(II)], [Fe3(C4H5N2)6]n. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1983, 39, 1212–1214. [Google Scholar] [CrossRef]
- Lin, J.-B.; Lin, R.-B.; Cheng, X.-N.; Zhang, J.-P.; Chen, X.-M. Solvent/additive-free synthesis of porous/zeolitic metal azolate frameworks from metal oxide/hydroxide. Chem. Commun. 2011, 47, 9185–9187. [Google Scholar] [CrossRef] [PubMed]
- Lanchas, M.; Vallejo-Sánchez, D.; Beobide, G.; Castillo, O.; Aguayo, A.T.; Luque, A.; Román, P. A direct reaction approach for the synthesis of zeolitic imidazolate frameworks: Template and temperature mediated control on network topology and crystal size. Chem. Commun. 2012, 48, 9930–9932. [Google Scholar] [CrossRef] [PubMed]
- Lanchas, M.; Arcediano, S.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yánez, S. Towards multicomponent MOFs via solvent-free synthesis under conventional oven and microwave assisted heating. Inorg. Chem. Front. 2015, 2, 425–433. [Google Scholar] [CrossRef]
- Julien, P.A.; Užarević, K.; Katsenis, A.D.; Kimber, S.A.J.; Wang, T.; Farha, O.K.; Zhang, Y.; Casaban, J.; Germann, L.; Etter, M.; et al. In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Huskić, I.; Christopherson, J.-C.; Užarević, K.; Friščić, T. In situ monitoring of vapour-induced assembly of pharmaceutical cocrystals using a benchtop powder X-ray diffractometer. Chem. Commun. 2016, 52, 5120–5123. [Google Scholar] [CrossRef] [PubMed]

| Liquid Additive | Solubility | Product | |
|---|---|---|---|
| caf | L-ta | ||
| none (neat grinding) | — | — | no reaction |
| water | 15 | 400 | caf hydrate |
| MeOH | 10 | 170 | cocrystal |
| EtOH | 6 | 72 | cocrystal |
| i-propanol | 3 | 27 | cocrystal |
| n-butanol | 3 | 16 | cocrystal |
| 2,2,2-trifluoroethanol | 450 | 2 | cocrystal + caf |
| benzene | <2 | <2 | cocrystal + caf |
| fluorobenzene | 15 | <2 | cocrystal + caf |
| acetonitrile | 21 | 13 | cocrystal |
| nitromethane | 45 | <2 | cocrystal |
| chloroform | 135 | <2 | cocrystal |
| ethyl acetate | 7 | 9 | cocrystal |
| di-i-propyl ether | <2 | <2 | cocrystal |
| ethylmethyl ketone | 8 | 15 | cocrystal |
| cyclohexane | <2 | <2 | no reaction |
| Solution or solvothermal synthesis | Advantages |
| • readily obtained single crystals | |
| • structural characterization by single crystal X-ray diffraction | |
| Limitations | |
| • requires soluble precursors | |
| • reagent dissolution requires heat and/or aggressive reagents (organic solvents, acids, bases) | |
| • large amounts of solvent waste generated | |
| • potentially hazardous handling of corrosive/explosive metal salts (chlorides, nitrates) in presence of organic liquids | |
| • reactions generate waste mineral acids or their salts (e.g., HNO3, HCl) | |
| • not applicable to products or reagents sensitive to heat or solvents | |
| Mechanochemistry by neat grinding | Advantages |
| • rapid synthesis (30–60 min) on multigram scale | |
| • quantitative yields when using metal salts as precursors | |
| • no reagent excess | |
| • no solvent waste | |
| • allows access to products and reactants sensitive to heat or solvents | |
| Limitations | |
| • structural characterization requires PXRD coupled with other techniques (e.g., solid-state NMR, thermal analysis, infrared spectroscopy) | |
| • neat milling may lead to unwanted product amorphization | |
| LAG mechanochemistry | Advantages |
| • rapid synthesis (30–60 min) on multigram scale | |
| • quantitative yields when using metal salts, carbonates and sometimes oxides | |
| • no solvent waste | |
| • no reagent excess | |
| • control of product topology by choice of liquid additive | |
| • allows access to products and reactants sensitive to heat or solvents | |
| • highly crystalline product | |
| Limitations | |
| • structural characterization requires PXRD coupled with other techniques (e.g., solid-state NMR, thermal analysis, infrared spectroscopy) | |
| ILAG mechanochemistry | Advantages |
| • rapid synthesis (30–60 min) on multigram scale | |
| • quantitative yields with metal salts, carbonates and oxides | |
| • no solvent waste | |
| • no reagent excess | |
| • control of product topology by choice of liquid or salt additives | |
| • allows access to products and reactants sensitive to heat or solvents | |
| • highly crystalline product | |
| Limitations | |
| • structural characterization requires PXRD coupled with other techniques (e.g., solid-state NMR, thermal analysis, infrared spectroscopy) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottillo, C.; Friščić, T. Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber. Molecules 2017, 22, 144. https://doi.org/10.3390/molecules22010144
Mottillo C, Friščić T. Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber. Molecules. 2017; 22(1):144. https://doi.org/10.3390/molecules22010144
Chicago/Turabian StyleMottillo, Cristina, and Tomislav Friščić. 2017. "Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber" Molecules 22, no. 1: 144. https://doi.org/10.3390/molecules22010144