A New Determination Method of the Solubility Parameter of Polymer Based on AIE
Abstract
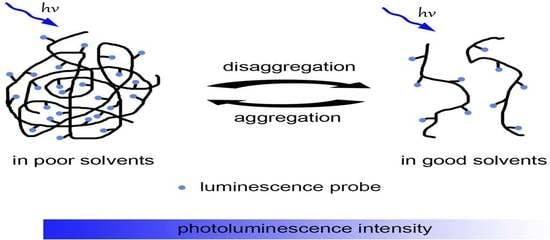
:1. Introduction
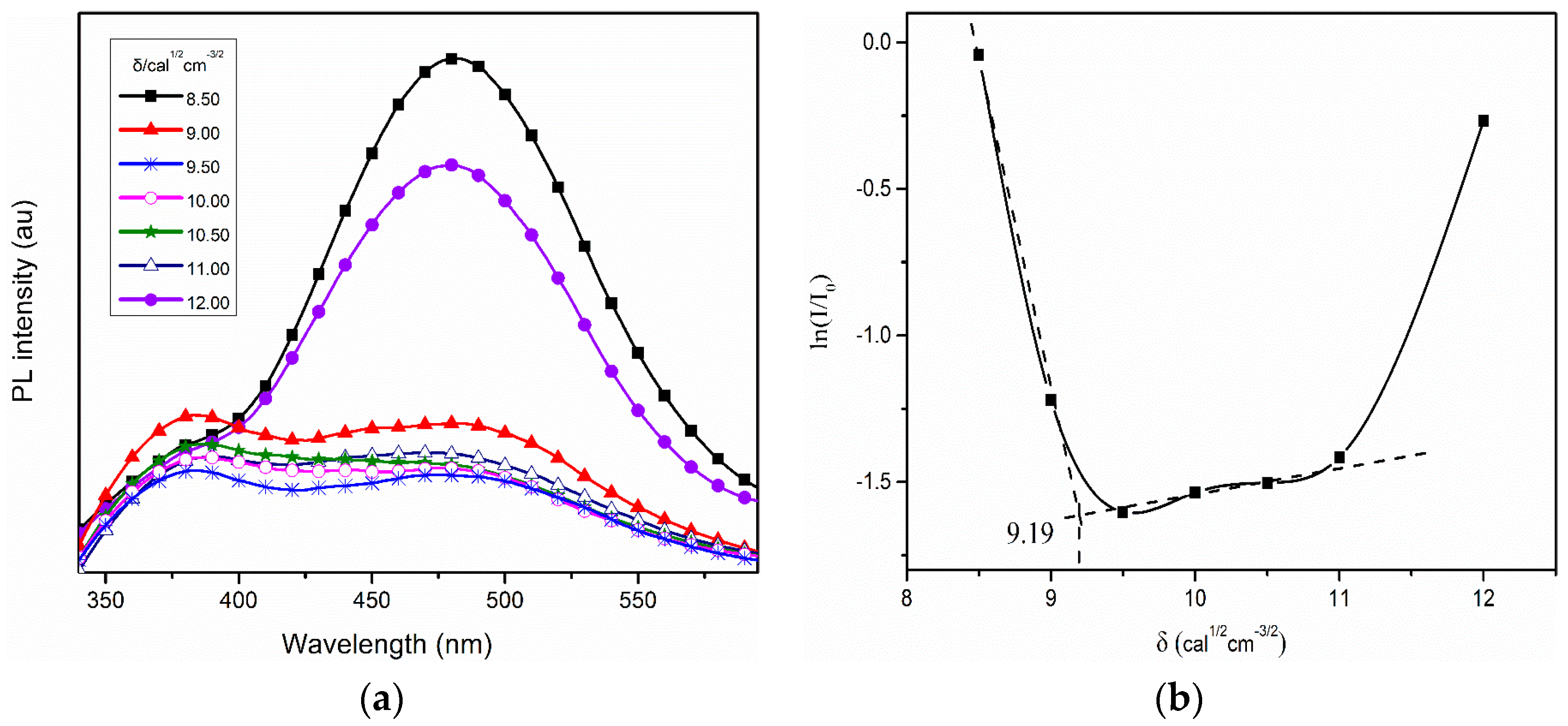
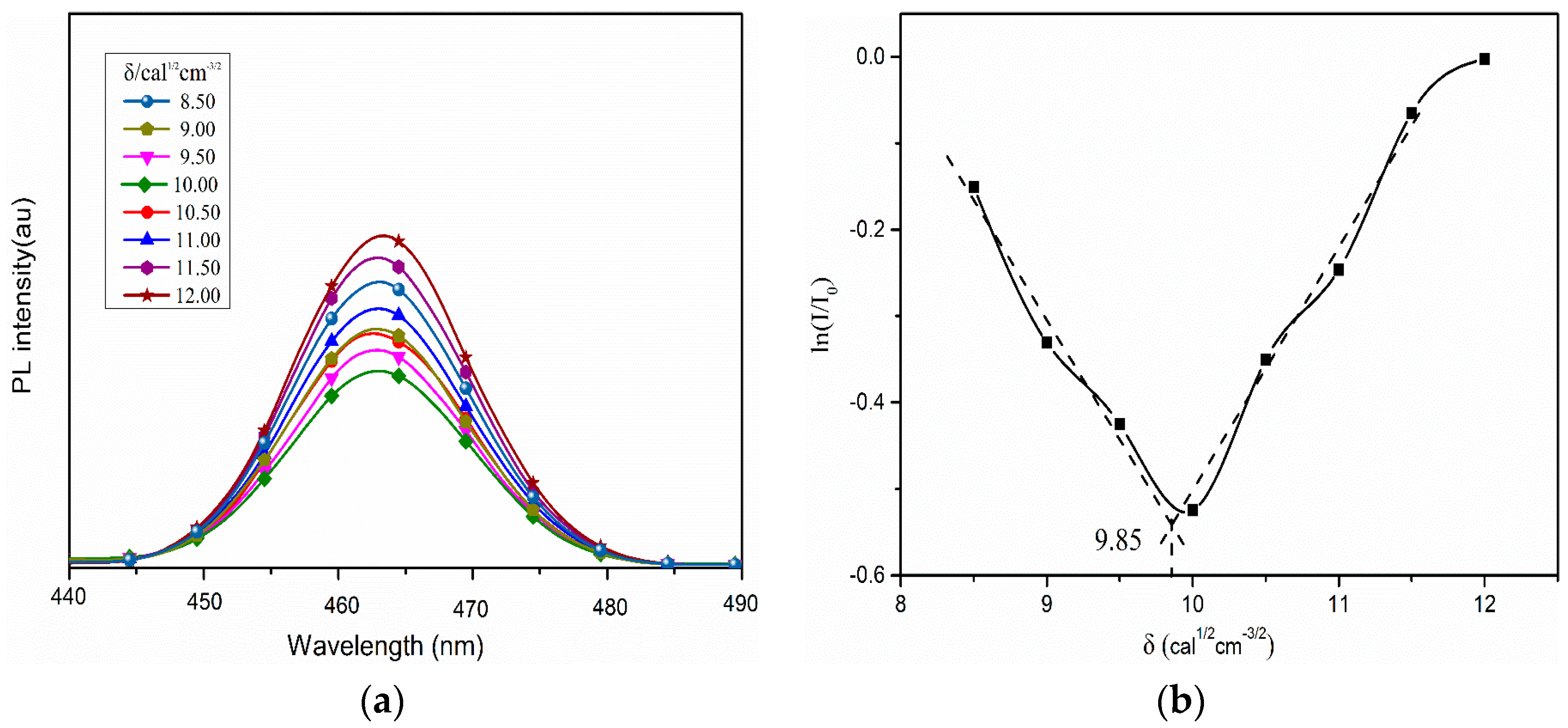
2. Results and Discussion
3. Materials and Methods
3.1. General
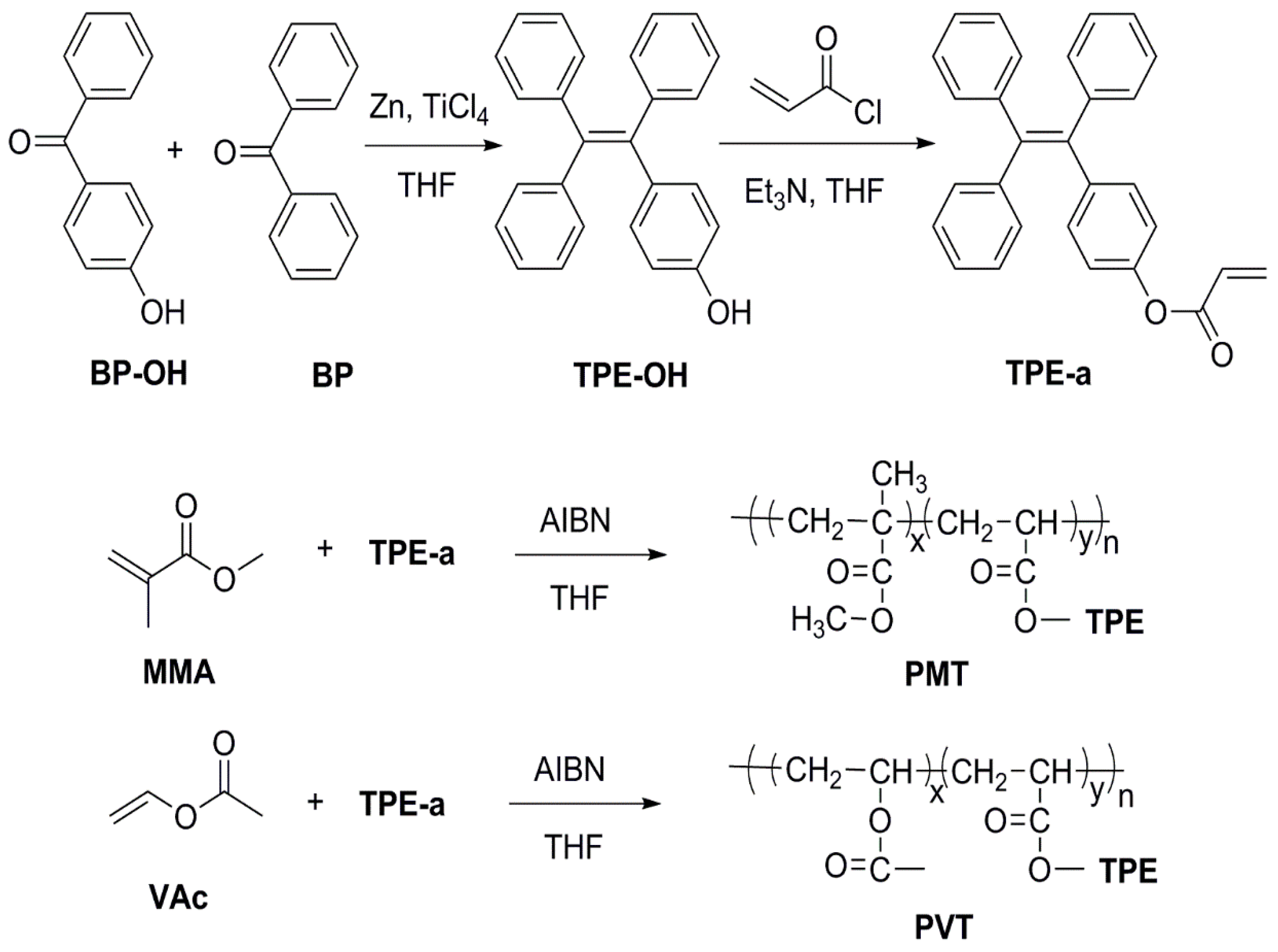
3.2. Synthesis of 4-Hydroxyl Tetraphenylethylene
3.3. Synthesis of 4-Acryloyl Tetraphenylethylene
3.4. Synthesis of PMT and PVT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonacchi, S.; Genovese, D.; Juris, R.; Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Luminescent silica nanoparticles: extending the frontiers of brightness. Angew. Chem. Int. Ed. 2011, 50, 4056–4066. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, S.; Ding, Y.; Pan, L.; Yang, H.; Jiang, B.; Yan, D.; Meng, Q. Fabrication and investigation of two-component film of 2,5-diphenyloxazole and octafluoronaphthalene exhibiting tunable blue/bluish violet fluorescence based on low vacuum physical vapor deposition method. J. Nanomater. 2016, 2016, 4363541. [Google Scholar] [CrossRef]
- Garcia-Sanchez, M.A.; Serratos, I.N.; Sosa, R.; Tapia-Esquivel, T.; Gonzalez-Garcia, F.; Rojas-Gonzalez, F.; Tello-Solis, S.R.; Palacios-Enriquez, A.Y.; Schulz, J.M.E.; Arrieta, A. Chlorophyll a covalently bonded to organo-modified translucent silica xerogels: optimizing fluorescence and maximum loading. Molecules 2016, 21, 961. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Photonic Research Systems. Available online: http://www.prsbio.com/index.html (accessed on 27 June 2016).
- Qiu, Z.; Han, T.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Polyarylcyanation of Diyne: A one-pot three-component convenient route for in situ generation of polymers with AIE characteristics. Macromolecules 2016, 49, 8888–8898. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.; Dong, Y.; Ren, Y.; Haeussler, M.; Lam, J.W.Y.; Wong, K.S.; Tang, B.Z. Color-Tunable, Aggregation-induced emission of a butterfly-shaped molecule comprising a pyran skeleton and two cholesteryl wings. J. Phys. Chem. B 2007, 111, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lam, J.W.Y.; Qin, A.; Liu, J.; Li, Z.; Tang, B.Z.; Sun, J.; Kwok, H.S. Aggregation-induced emissions of tetraphenylethene derivatives and their utilities as chemical vapor sensors and in organic light-emitting diodes. Appl. Phys. Lett. 2007, 91, 011111. [Google Scholar] [CrossRef]
- Howell, J.S.; Stephens, B.O.; Boucher, D.S. Convex solubility parameters for polymers. J. Polym. Sci. B Polym. Phys. 2015, 53, 1089–1097. [Google Scholar] [CrossRef]
- Ng, S.C.; Chee, K.K. Solubility parameters of copolymers as determined by turbidimetry. Eur. Polym. J. 1997, 33, 749–752. [Google Scholar] [CrossRef]
- Imaizumi, M.; Nagata, T.; Goto, Y.; Okino, Y.; Takahashi, T.; Koyama, K. Solubility parameters of biodegradable polymers from turbidimetric titrations. Kobunshi Ronbunshu 2005, 62, 438–440. [Google Scholar] [CrossRef]
- Minghetti, P.; Cilurzo, F.; Casiraghi, A.; Montanari, L. Application of viscometry and solubility parameters in miconazole patches development. Int. J. Pharm. 1999, 190, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Cavus, S.; Cakal, E.; Sevgili, L.M. Solvent dependent swelling behaviour of poly(N-vinylcaprolactam) and poly(N-vinylcaprolactam-co-itaconic acid) gels and determination of solubility parameters. Chem. Pap. 2015, 69, 1367–1377. [Google Scholar] [CrossRef]
- Yang, T.-H.; Lue, S.J. Modeling sorption behavior for ethanol/water mixtures in a cross-linked polydimethylsiloxane membrane using the Flory-Huggins equation. J. Macromol. Sci. B 2013, 52, 1009–1029. [Google Scholar] [CrossRef]
- Krishna, R. Describing mixture permeation across polymeric membranes by a combination of Maxwell-Stefan and Flory-Huggins models. Polymer 2016, 103, 124–131. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Hildebrand, J.H. The Solubility of Non-Electrolytes; Reinhold: New York, NY, USA, 1936. [Google Scholar]
- Plater, M.J.; Jackson, T. Polyaromatic amines. Part 3: Synthesis of poly(diarylamino)styrenes and related compounds. Tetrahedron 2003, 59, 4673–4685. [Google Scholar] [CrossRef]
- Sample Availability: Not available.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Huang, T.Y.; Wang, K.M.; Tang, B.Z.; Yu, Q. A New Determination Method of the Solubility Parameter of Polymer Based on AIE. Molecules 2017, 22, 54. https://doi.org/10.3390/molecules22010054
Jiang S, Huang TY, Wang KM, Tang BZ, Yu Q. A New Determination Method of the Solubility Parameter of Polymer Based on AIE. Molecules. 2017; 22(1):54. https://doi.org/10.3390/molecules22010054
Chicago/Turabian StyleJiang, Shan, Tian Ya Huang, Ke Min Wang, Ben Zhong Tang, and Qiang Yu. 2017. "A New Determination Method of the Solubility Parameter of Polymer Based on AIE" Molecules 22, no. 1: 54. https://doi.org/10.3390/molecules22010054