A Fluorescent Coumarin-Based Probe for the Fast Detection of Cysteine with Live Cell Application
Abstract
:1. Introduction
2. Results and Discussion
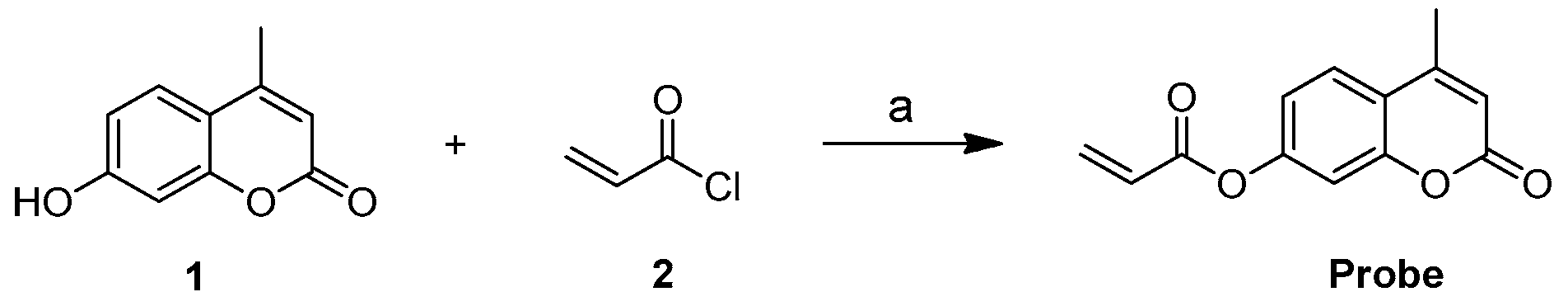
2.1. Characterization of the Probe
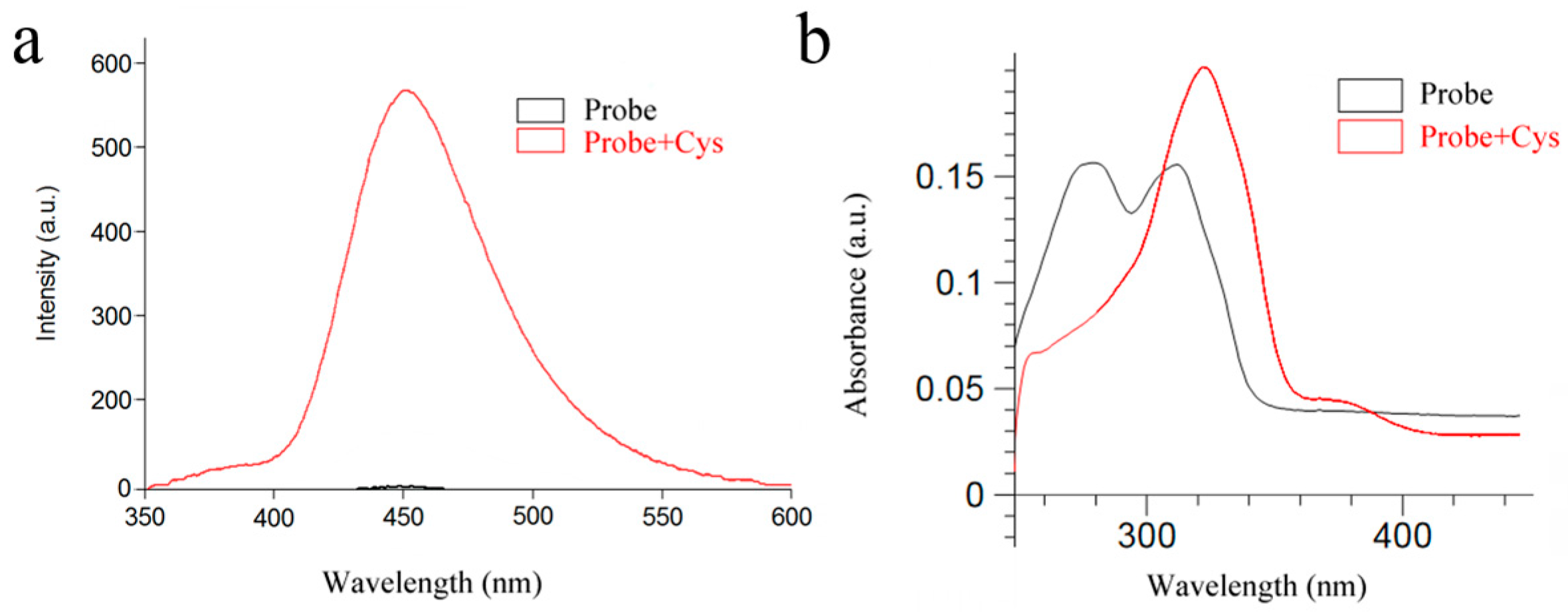
2.2. UV-Vis Absorption and Fluorescence Spectra
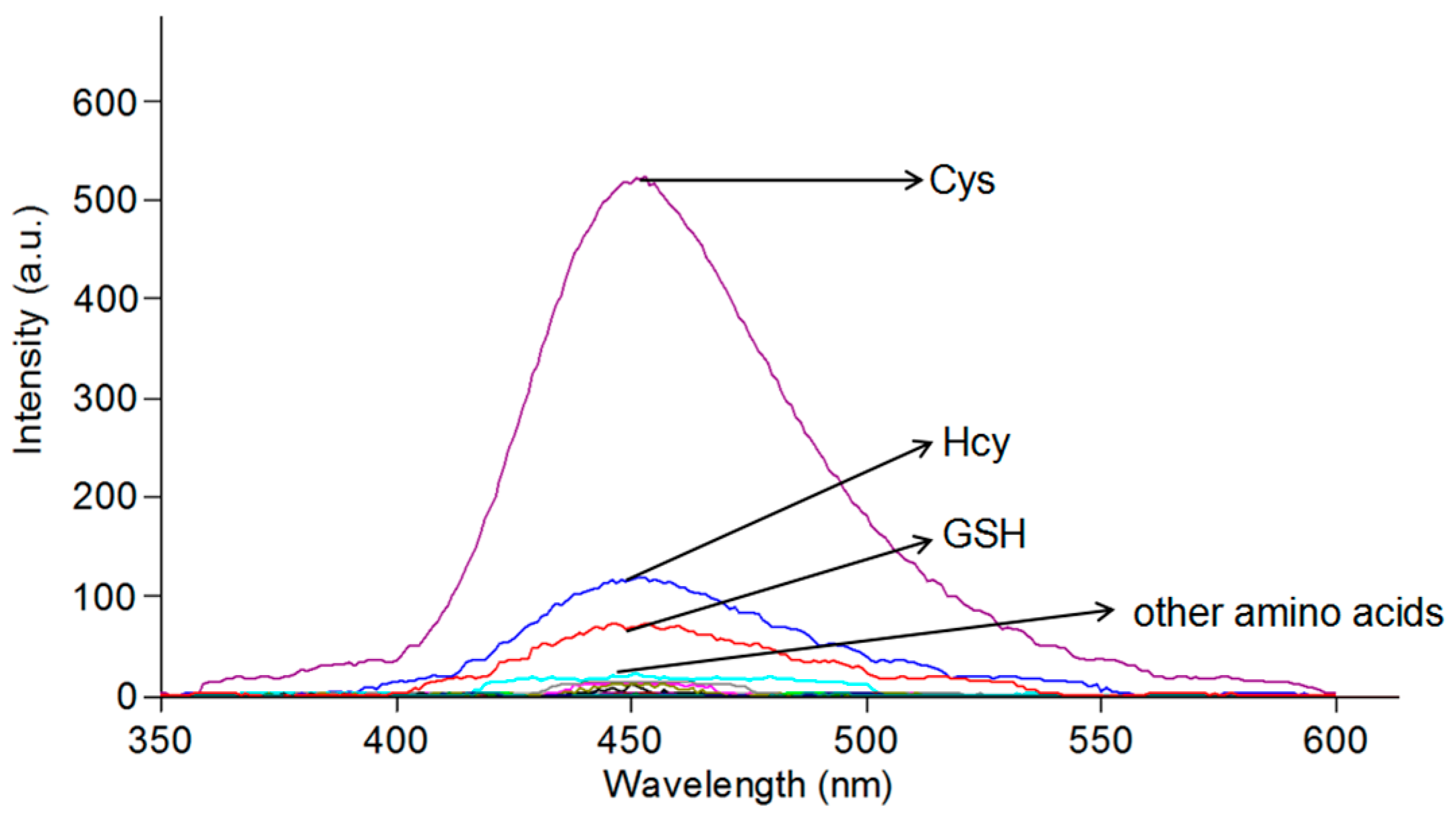
2.3. Selectivity of the Probe for Cys
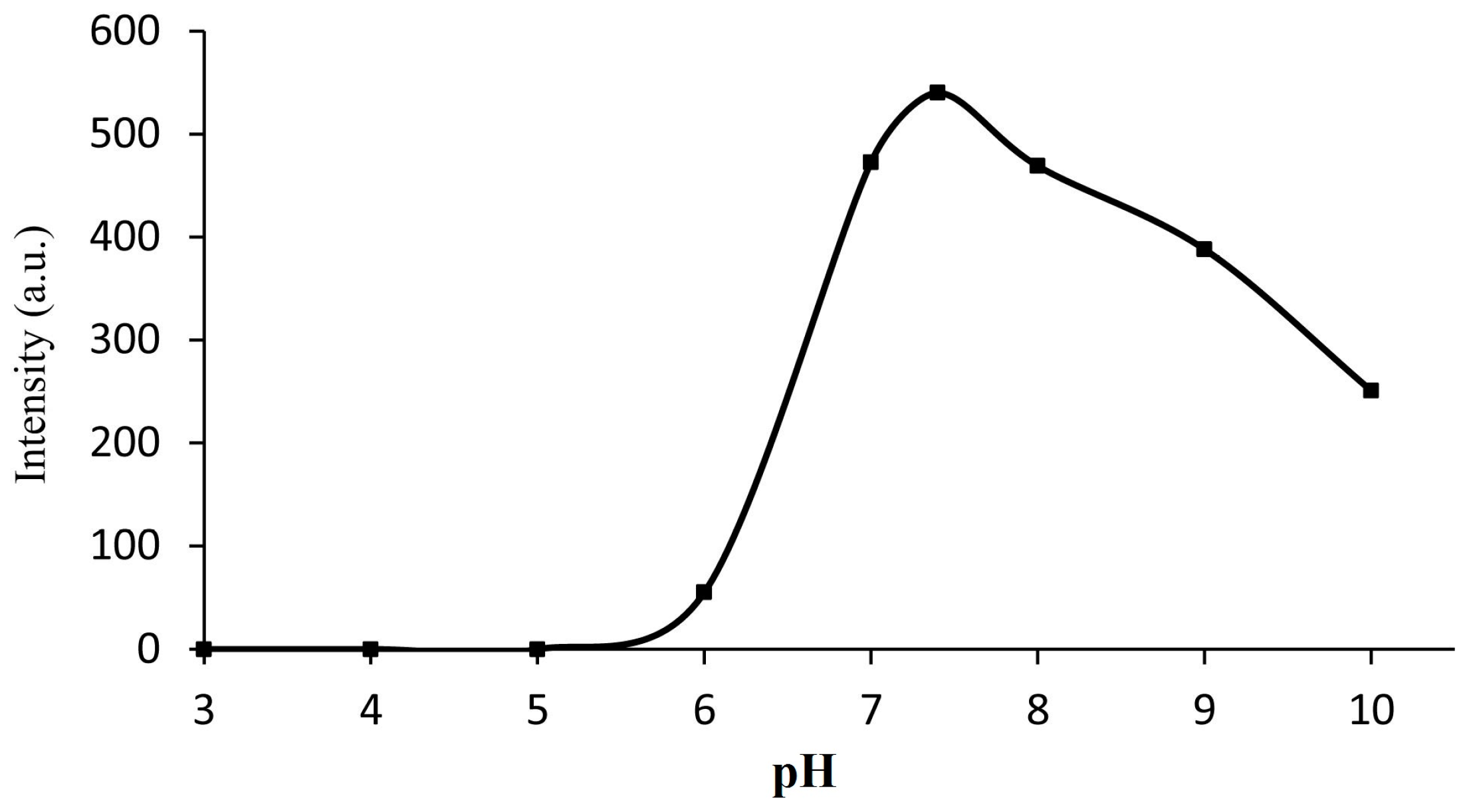
2.4. Effect of pH on the Fluorescence Response of Probe
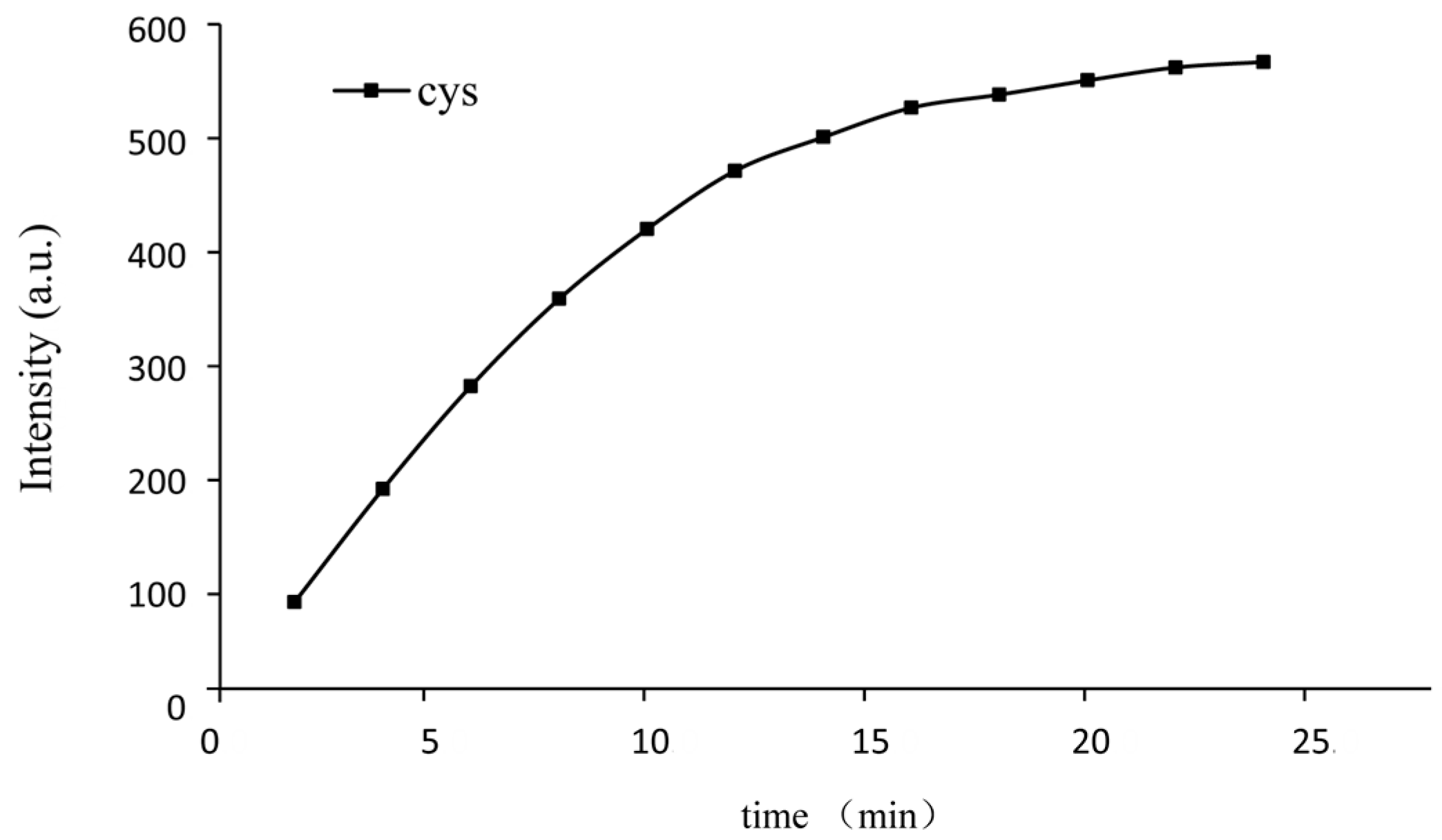
2.5. Effect of Reaction Time
2.6. Quantitative Responses of the Probe for Cys
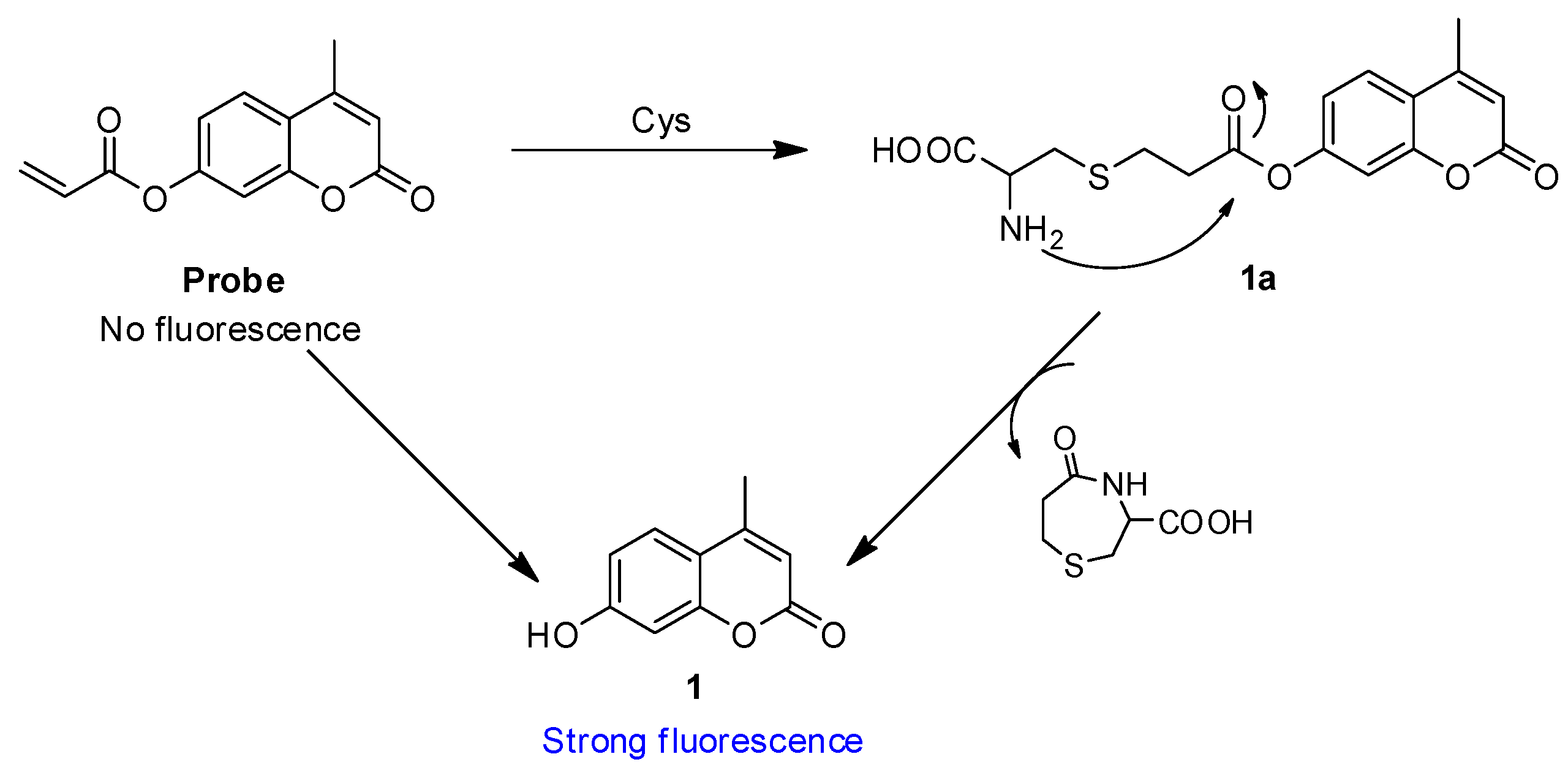
2.7. Reaction Mechanism
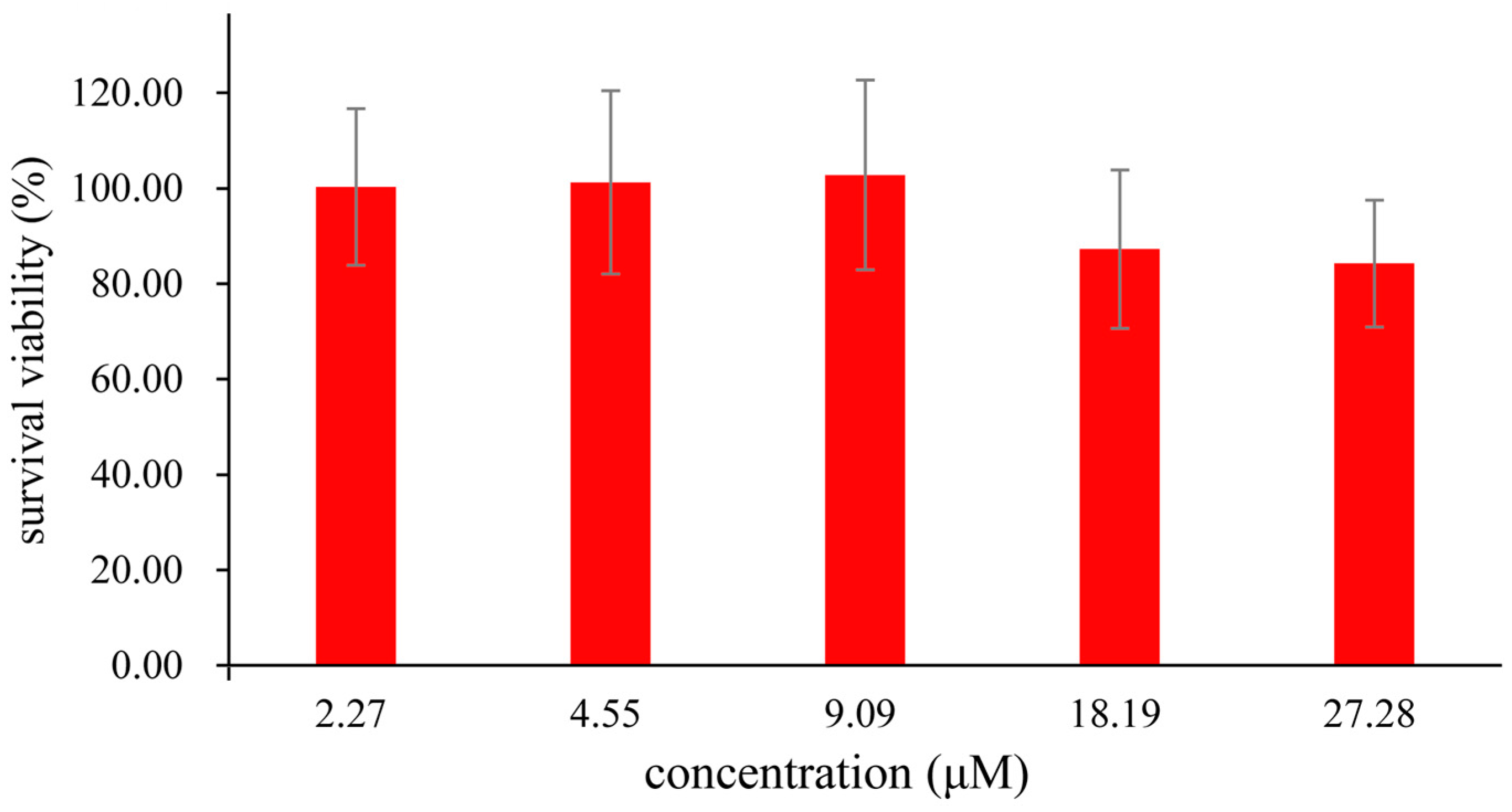
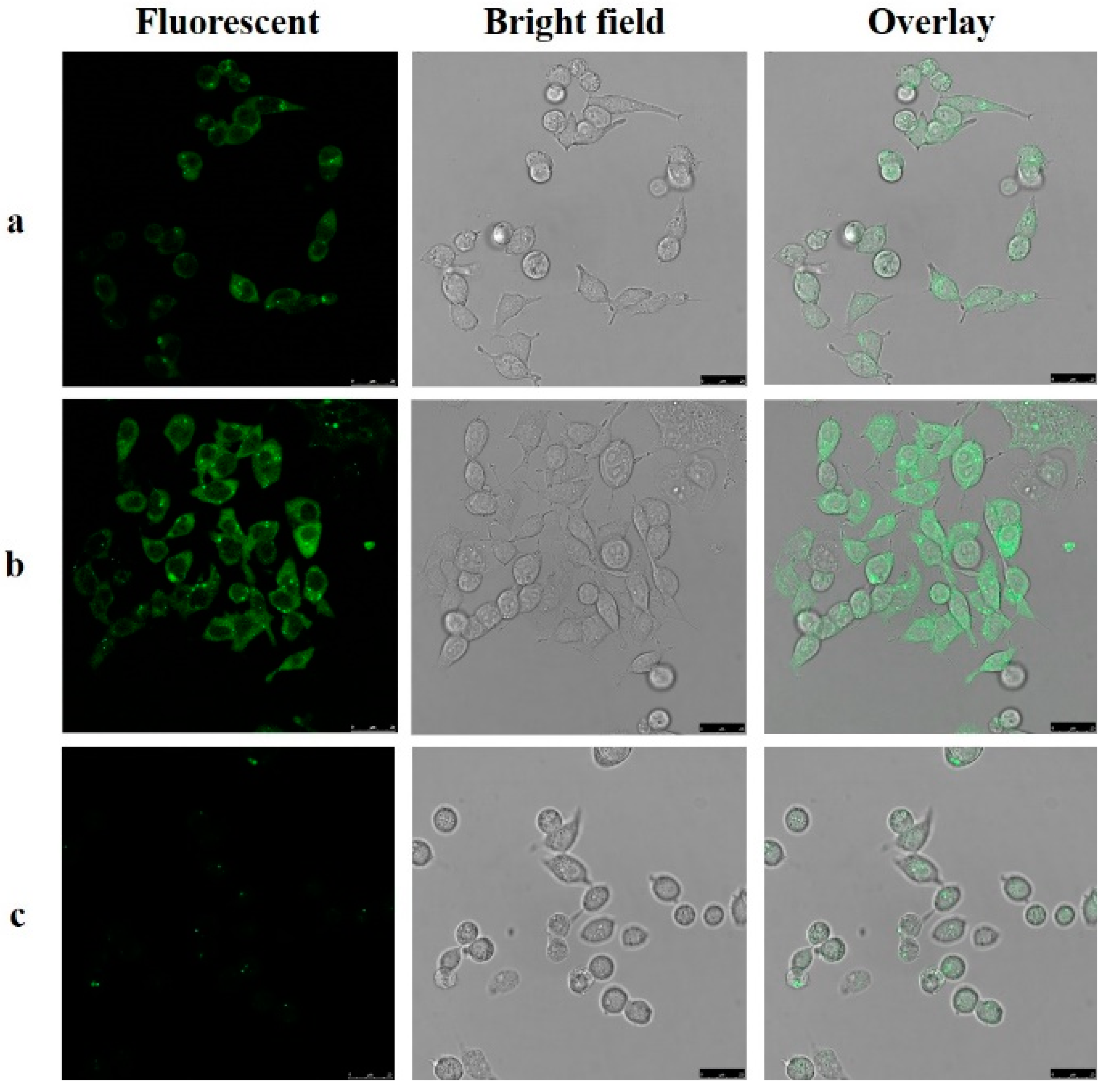
2.8. Application of the Probe
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis of the Probe (4-Methyl-2-oxo-2H-chromen-7-yl Acrylate)
3.3. Absorption and Fluorescence Spectroscopy
3.4. Cell Culture for HepG2
3.5. Fluorescence Imaging of Cys in Living Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bragoszewski, P.; Wasilewski, M.; Sakowska, P.; Gornicka, A.; Bottinger, L.; Qiu, J.; Wiedemann, N.; Chacinska, A. Retro-translocation of mitochondrial intermembrane space proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 7713–7718. [Google Scholar] [CrossRef] [PubMed]
- Abby, S.L.; Harris, I.M.; Harris, K.M. Homocysteine and cardiovascular disease. Curr. Atheroscler. Rep. 2004, 6, 101–106. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Kanda, T.; Sato, K.; Takaishi, M.; Nakajima, K.; Yamamoto, M.; Kamijima, R.; Digiovanni, J.; Sano, S. Cathepsin K is involved in development of psoriasis-like skin lesions through TLR-dependent Th17 activation. J. Immunol. 2013, 190, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.Y.M.; Hoenderop, J.G.J.; Bindels, R.J.M. Calpain-3-mediated regulation of the Na+-Ca2+ exchanger isoform 3. Pflugers Arch. 2016, 468, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Huo, F.; Zhang, J.; Martínez-Máñez, R.; Yang, Y.; Lv, H.; Li, S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huo, F.; Yin, C.; Zheng, A.; Chao, J.; Li, Y.; Nie, Z.; Martinez-Manez, R.; Liu, D. Thiol-chromene click chemistry: A coumarin-based derivative and its use as regenerable thiol probe and in bioimaging applications. Biosens. Bioelectron. 2013, 47, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Harfield, J.C.; Batchelor-Mcauley, C.; Compton, R.G. Electrochemical determination of glutathione: A review. Analyst 2012, 137, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. ACC Curr. J. Rev. 2002, 11, 30. [Google Scholar] [CrossRef]
- Xue, S.; Ding, S.; Zhai, Q.; Zhang, H.; Feng, G. A readily available colorimetric and near-infrared fluorescent turn-on probe for rapid and selective detection of cysteine in living cells. Biosens. Bioelectron. 2015, 68, 316. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Tang, L.Y.; Cen, Y.L.; Lin, Y.; Su, F.X.; Jia, W.H.; Ren, Z.F. Interaction of body mass index and a polymorphism in gene of catalytic subunit of glutamate-cysteine ligase on breast cancer risk among Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi 2013, 34, 1115. [Google Scholar] [PubMed]
- Roy, N.; Nazeem, P.A.; Babu, T.D.; Abida, P.S.; Narayanankutty, A.; Valsalan, R.; Valsala, P.A.; Raghavamenon, A.C. EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-l-Cysteine Sulfoxide. Interdiscip. Sci. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Li, C.Y.; Ding, Y.; Chau, D.; Li, G.; Kung, H.F.; Lin, M.C.; Peng, Y. Recombinant adeno-associated virus mediated RNA interference inhibits metastasis of nasopharyngeal cancer cells in vivo and in vitro by suppression of Epstein-Barr virus encoded LMP-1. Int. J. Oncol. 2006, 4, 595–603. [Google Scholar] [CrossRef]
- Geng, P.; Yao, J.; Zhu, Y. Hogg1 Ser326Cys polymorphism and lung cancer susceptibility: A meta-analysis. Mol. Biol. Rep. 2014, 41, 2299. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, J.C.; Cheng, T.H.; Yuan, S.S.; Wang, Y.M. Sensitivity evaluation of NBD-SCN towards cysteine/homocysteine and its bioimaging applications. Biosens. Bioelectron. 2014, 56, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Zhu, H.; Wang, H.; Zhang, H.S. Determination of thiol compounds by HPLC and fluorescence detection with 1,3,5,7-tetramethyl-8-bromomethyl-difluoroboradiaza-S-indacene. J. Sep. Sci. 2013, 36, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Iizuka, H.; Isokawa, M.; Tsunoda, M.; Ichiba, H.; Sadamoto, K.; Fukushima, T. HPLC-fluorescence determination of thiol compounds in the serum of human male and female subjects using HILIC-mode column. Biomed. Chromatogr. 2014, 28, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Capehart, S.L.; Carlson, E.E. Mass spectrometry-based assay for the rapid detection of thiol-containing natural products. Chem. Commun. 2016, 52, 13229. [Google Scholar] [CrossRef] [PubMed]
- García-Santamarina, S.; Boronat, S.; Domènech, A.; Ayté, J.; Molina, H.; Hidalgo, E. Monitoring in vivo reversible cysteine oxidation in proteins using ICAT and mass spectrometry. Nat. Protoc. 2014, 9, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.-J.; Sun, Y.-Q.; Su, J.; Chao, J.-B.; Zhi, H.-J.; Yin, C.-X. Colorimetric detection of thiols using a chromene molecule. Org. Lett. 2009, 11, 4918–4921. [Google Scholar] [CrossRef] [PubMed]
- Kruusma, J.; Benham, A.M.; Williams, J.A.; Kataky, R. An introduction to thiol redox proteins in the endoplasmic reticulum and a review of current electrochemical methods of detection of thiols. Analyst 2006, 131, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Vandeberg, P.J.; Johnson, D.C. Pulsed electrochemical detection of cysteine, cystine, methionine, and glutathione at gold electrodes following their separation by liquid chromatography. Anal. Chem. 2002, 65, 2713–2718. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, L.; Wang, J. Miniaturized capillary electrophoresis system with a carbon nanotube microelectrode for rapid separation and detection of thiols. Talanta 2004, 64, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Nam, S.W.; Park, S.; Yoon, J. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging. Chem. Sci. 2012, 3, 2760–2765. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zhao, S.; Gao, W.; Chen, B.; He, L.; Zhu, S. A unique approach to development of near-infrared fluorescent sensors for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 13510. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xing, Y.; Li, P.; Zhang, N.; Yu, F.; Yang, G. A rhodamine-based fluorescent probe containing a Se-N bond for detecting thiols and its application in living cells. J. Am. Chem. Soc. 2007, 129, 11666–11667. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Duan, H.; Wang, J.; Yang, Z.; Lin, Y. Aggregation-enhanced excimer emission (AEEE) based on pyrenylchalcone and 2-to-4 molecular decoder by biothiols and polyanions in aqueous media. Sens. Actuators B Chem. 2014, 195, 80–84. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, M.; Lei, T.; Liu, X.; Zhang, Q.; Zong, C. An indicator-displacement assay based on the Murexide-Hg2+ system for fluorescence turn-on detection of biothiols in biological fluids. Sens. Actuators B Chem. 2017, 249, 90–95. [Google Scholar] [CrossRef]
- Mei, J.; Wang, Y.; Tong, J.; Wang, J.; Qin, A.; Sun, J.Z.; Tang, B.Z. Discriminatory detection of cysteine and homocysteine based on dialdehyde-functionalized aggregation-induced emission fluorophores. Chemistry 2013, 19, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, Q.; Wang, N.; Chen, Z.; Zhang, W.; Qian, J. A fluorescent probe for the discrimination between Cys and GSH. Anal. Methods 2015, 7, 10371–10375. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.F.; Yang, S.H.; Yang, W.C.; Yang, G.F. A coumarin-based fluorescent probe for selective and sensitive detection of thiophenols and its application. Anal. Chem. 2014, 86, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Long, L.; Yuan, L.; Cao, Z.; Chen, B.; Tan, W. A ratiometric fluorescent probe for cysteine and homocysteine displaying a large emission shift. Org. Lett. 2008, 10, 5577. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, D.N.; Kim, H.J. Highly selective fluorescent sensor for homocysteine and cysteine. Tetrahedron Lett. 2008, 49, 4879–4881. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, T.K.; Lee, J.H.; Kim, H.J.; Hong, J.I. Fluorescence turn-on probe for homocysteine and cysteine in water. Chem. Commun. 2008, 46, 6173. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Pradhan, T.; Han, J.H.; Heo, K.J.; Lee, J.H.; Kang, C.; Kim, J.S. Molecular modulated cysteine-selective fluorescent probe. Biomaterials 2012, 33, 8495–8502. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Han, J.H.; Pradhan, T.; Kim, S.; Lee, S.W.; Sessler, J.L.; Kim, T.W.; Kang, C.; Kim, J.S. A cysteine-selective fluorescent probe for the cellular detection of cysteine. Biomaterials 2012, 33, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.J.; Sun, Y.Q.; Su, J.; Yang, Y.T.; Yin, C.X.; Chao, J.B. Chromene “lock”, thiol “key”, and mercury(II) ion “hand”: A single molecular machine recognition system. Org. Lett. 2010, 12, 4756–4759. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.M.; Hrycyna, C.A.; Chmielewski, J. Bivalent probes of the human multidrug transporter P-glycoprotein. Biochemistry 2006, 45, 11695–11702. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsuno, H.; Ushida, M.; Katayama, K.; Saeki, K.; Itoh, N. 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman’s reagent in thiol-quantification enzyme assays. Angew. Chem. 2005, 44, 2922–2925. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fu, Q.; Fan, H.; Ho, J.; Wang, W. A highly selective fluorescent probe for thiophenols. Angew. Chem. 2007, 46, 8445–8448. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Y.; Strongin, R.M. Conjugate addition/cyclization sequence enables selective and simultaneous fluorescence detection of cysteine and homocysteine. Angew. Chem. 2011, 50, 10690–10693. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-W.; Wang, M.-Y.; Yin, D.; Zhang, X. Facile preparation of naphthol AS-based fluorescent probe for highly selective detection of cysteine in aqueous solution and its imaging application in living cells. Sens. Actuators B Chem. 2017, 248, 332–337. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, K.; Tian, B.; Zhang, J. A fluorescein-based fluorescence probe for the fast detection of thiol. Tetrahedron Lett. 2016, 57, 2478–2483. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, T.; Liu, Y.Z.; Yan, T.; Li, Y.; Miao, J.Y.; Zhao, B.X. A ratiometric fluorescent probe for cysteine and its application in living cells. Sens. Actuators B Chem. 2015, 207, 872–877. [Google Scholar] [CrossRef]
- Liao, Y.C.; Venkatesan, P.; Wei, L.F.; Wu, S.P. A coumarin-based fluorescent probe for thiols and its application in cell imaging. Sens. Actuators B Chem. 2016, 232, 732–737. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Q.; Yao, Y.; Fan, X.; Zhang, W.; Qian, J. Highly sensitive detection of cysteine over glutathione and homo-cysteine: New insight into the Michael addition of mercapto group to maleimide. Biosens. Bioelectron. 2017, 91, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, W.; Xu, C.; Liu, W. A colorimetric and fluorescent probe for detecting intracellular biothiols. Biosens. Bioelectron. 2016, 85, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, H.; Yin, B. A colorimetric and ratiometric fluorescent probe for selective detection and cellular imaging of glutathione. Biosens. Bioelectron. 2015, 72, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yin, P.; Shen, Y.; Zhang, L.; Deng, J.; Xue, S.; Li, H.; Guo, B.; Zhang, Y.; Yao, S. A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem. Commun. 2013, 49, 4640–4642. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Zhu, M.; Fan, S.; Wang, Z.; Wu, X.; Tang, J.; Liu, J.; Wang, Y.; Hua, R. A simple and effective ratiometric fluorescent probe for the selective detection of cysteine and homocysteine in aqueous media. Molecules 2016, 21, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.F.; Thirumalaivasan, N.; Liao, Y.C.; Wu, S.P. Fluorescent coumarin-based probe for cysteine and homocysteine with live cell application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, Y.; Wang, B.; Jiang, Y. A sensitive fluorescent probe based on coumarin for detection of cysteine in living cells. J. Photochem. Photobiol. A Chem. 2017, 338, 178–182. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Li, N.; Huang, J.; Wang, Q.; Gu, Y. A novel DCM-NBD conjugate fluorescent probe for discrimination of Cys/Hcy from GSH and its bioimaging applications in living cells and animals. Sens. Actuators B Chem. 2017, 245, 297–304. [Google Scholar] [CrossRef]
- Nagaraja, D.; Melavanki, R.M.; Patil, N.R.; Kusanur, R.A. Solvent effect on the relative quantum yield and fluorescence quenching of 2DAM. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 122–128. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Reference | Response Time | Stokes Shift | Detection Limit | Selectivity for Cys |
|---|---|---|---|---|
| Sensors and Actuators B, 2017 [46] | 12 min | 122 nm | 17.1 nM | no |
| Sensors and Actuators B, 2016 [47] | 3 h | 110 nm | 192 nM | no |
| Biosensors and Bioelectronics, 2017 [48] | 20 min | 85 nm | 14 nM | yes |
| Biosensors and Bioelectronics, 2016 [49] | 30 min | 96 nm | 0.874 μM | no |
| Biosensors and Bioelectronics, 2014 [50] | 40 min | 55 nm | 0.657 μM | yes |
| ChemComm, 2013 [51] | 2 h | 97 nm | - | no |
| Molecules, 2016 [52] | 2.5 h | 174 nm | 0.911 μM | no |
| This work | 30 min | 125 nm | 47.7 nM | yes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, R.-F.; Lan, J.-S.; Li, X.-D.; Liang, H.-F.; Liao, Y.; Lu, Y.-J.; Zhang, T.; Ding, Y. A Fluorescent Coumarin-Based Probe for the Fast Detection of Cysteine with Live Cell Application. Molecules 2017, 22, 1618. https://doi.org/10.3390/molecules22101618
Zeng R-F, Lan J-S, Li X-D, Liang H-F, Liao Y, Lu Y-J, Zhang T, Ding Y. A Fluorescent Coumarin-Based Probe for the Fast Detection of Cysteine with Live Cell Application. Molecules. 2017; 22(10):1618. https://doi.org/10.3390/molecules22101618
Chicago/Turabian StyleZeng, Rui-Feng, Jin-Shuai Lan, Xiao-Die Li, Hui-Fen Liang, Yan Liao, Ying-Jie Lu, Tong Zhang, and Yue Ding. 2017. "A Fluorescent Coumarin-Based Probe for the Fast Detection of Cysteine with Live Cell Application" Molecules 22, no. 10: 1618. https://doi.org/10.3390/molecules22101618





