Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation of the Caco-2 Cell Monolayer
2.2. Validation of High Performance Liquid Chromatography Analysis Method
2.3. Bidirectional Transport Determination
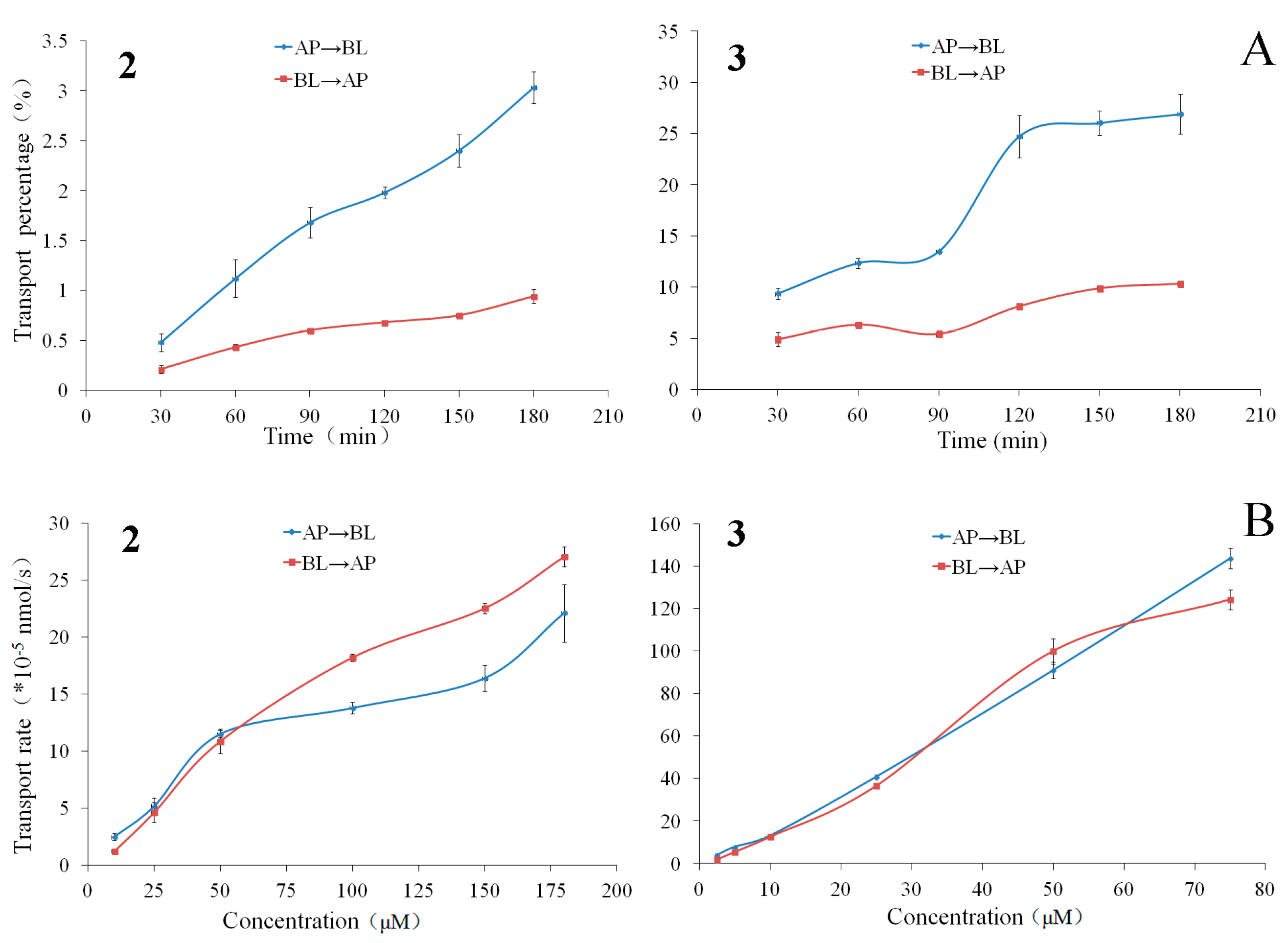
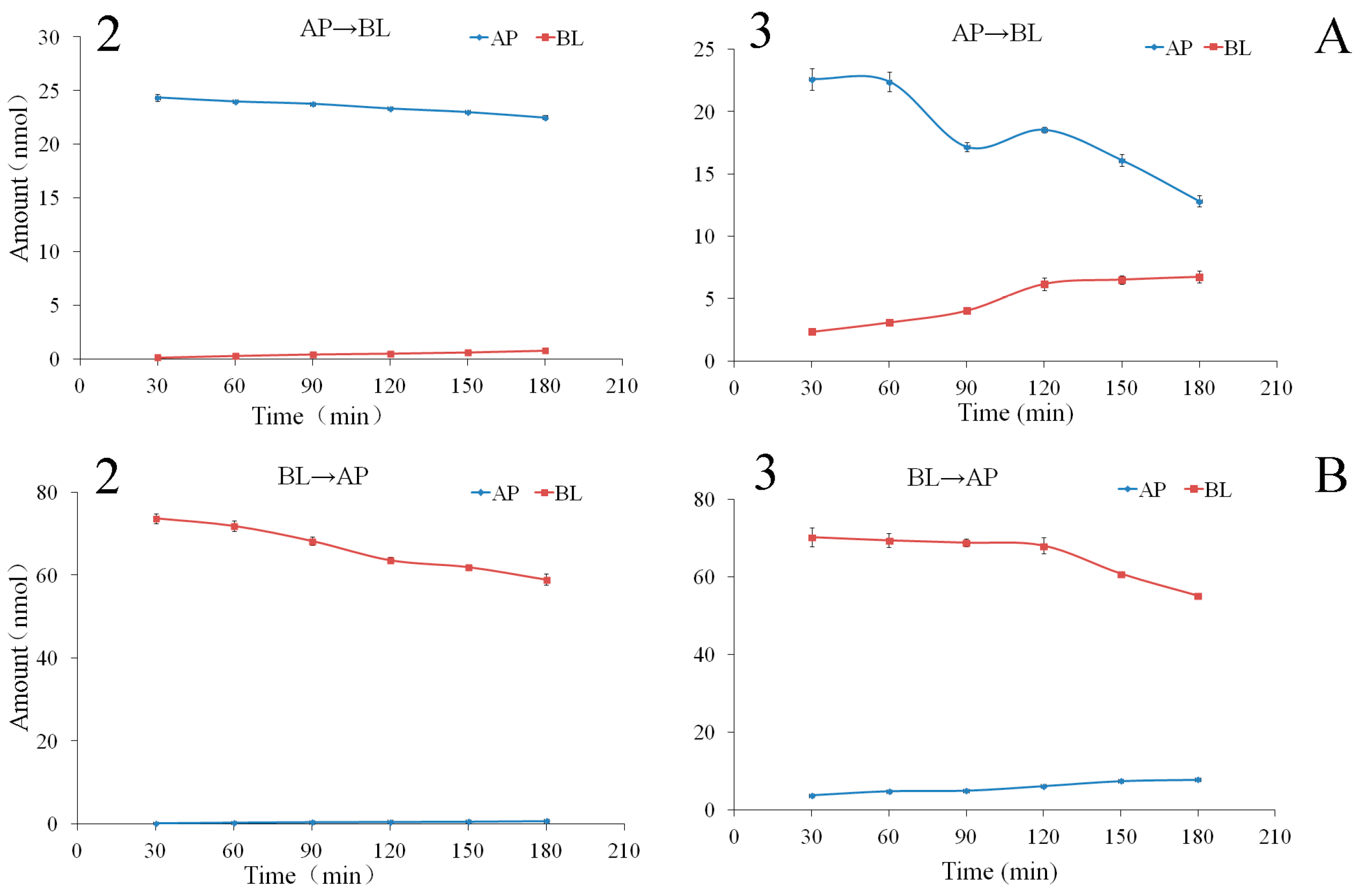
2.3.1. Bidirectional Transport of Triterpenoids 1–3
2.3.2. The Time Course and Concentration-Dependence of Permeation of Triterpenoids 2 and 3
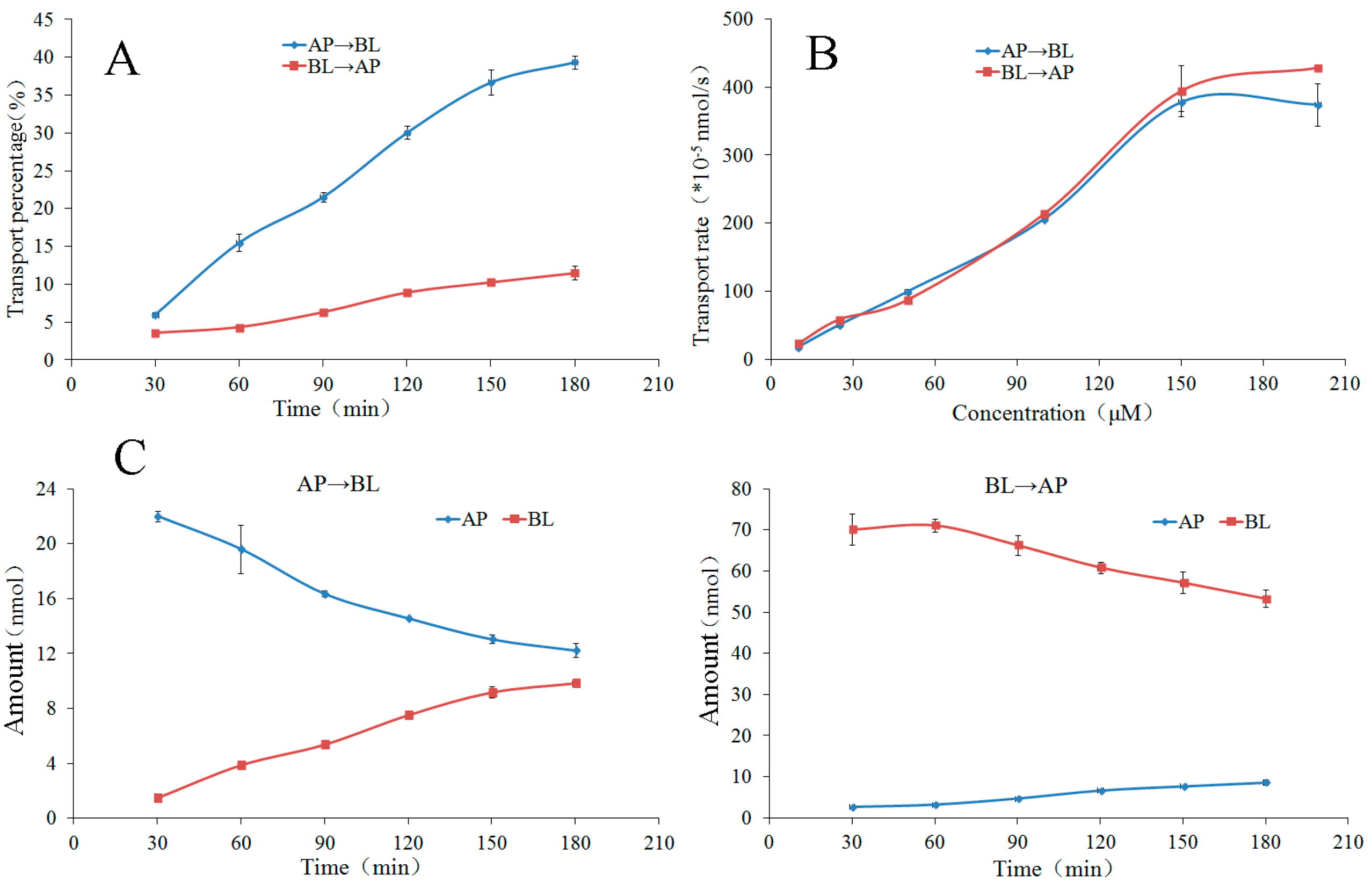
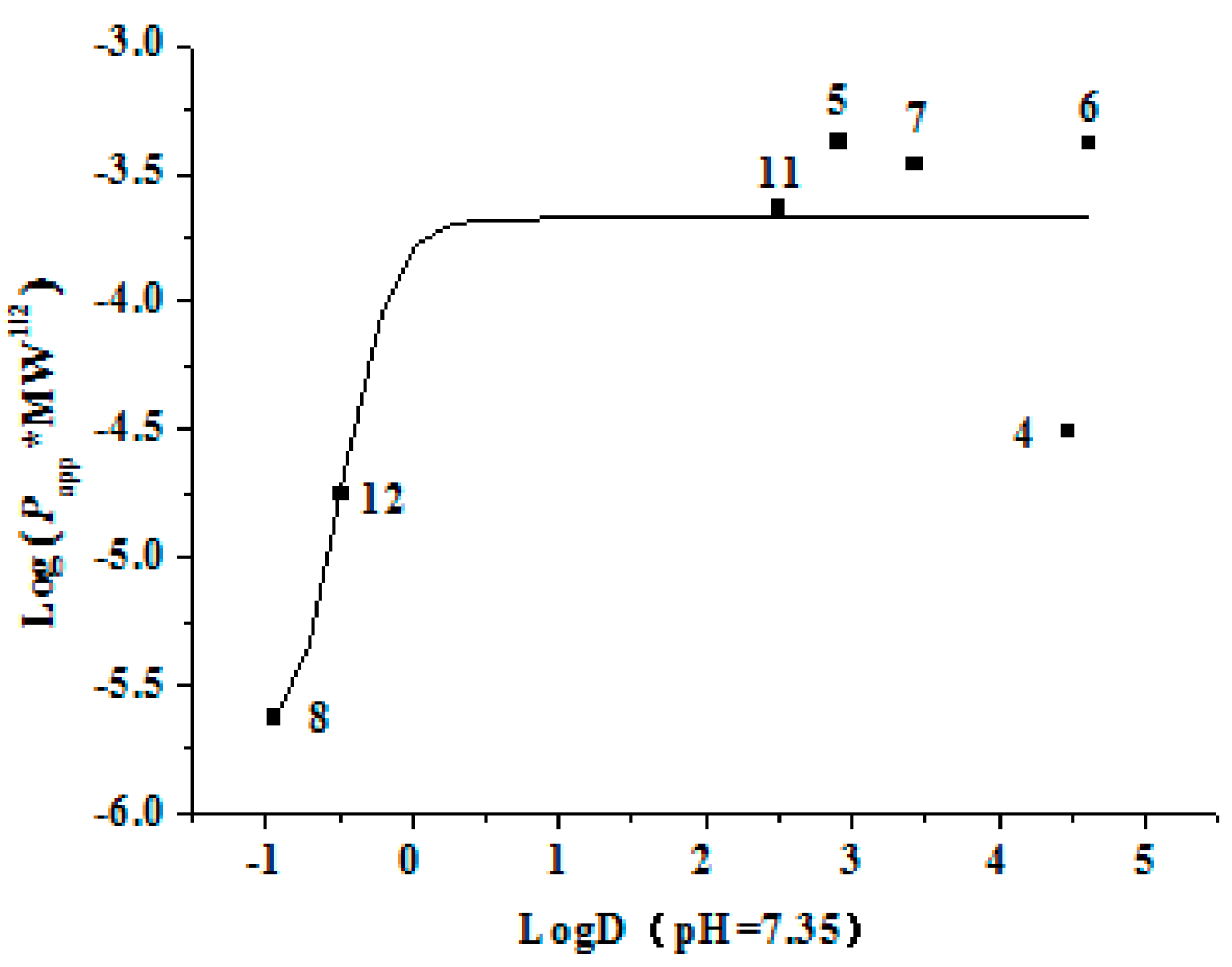
2.3.3. Bidirectional Transport of Chalcones 4–8
2.3.4. The Time Course and Concentration-Dependence of Membrane Permeation of Chalcones 4–7
2.3.5. Bidirectional Transport of Flavonones 9 and 10
2.3.6. The Time Course and Concentration-Dependence of Permeation of Flavonone 9
3. Experimental Section
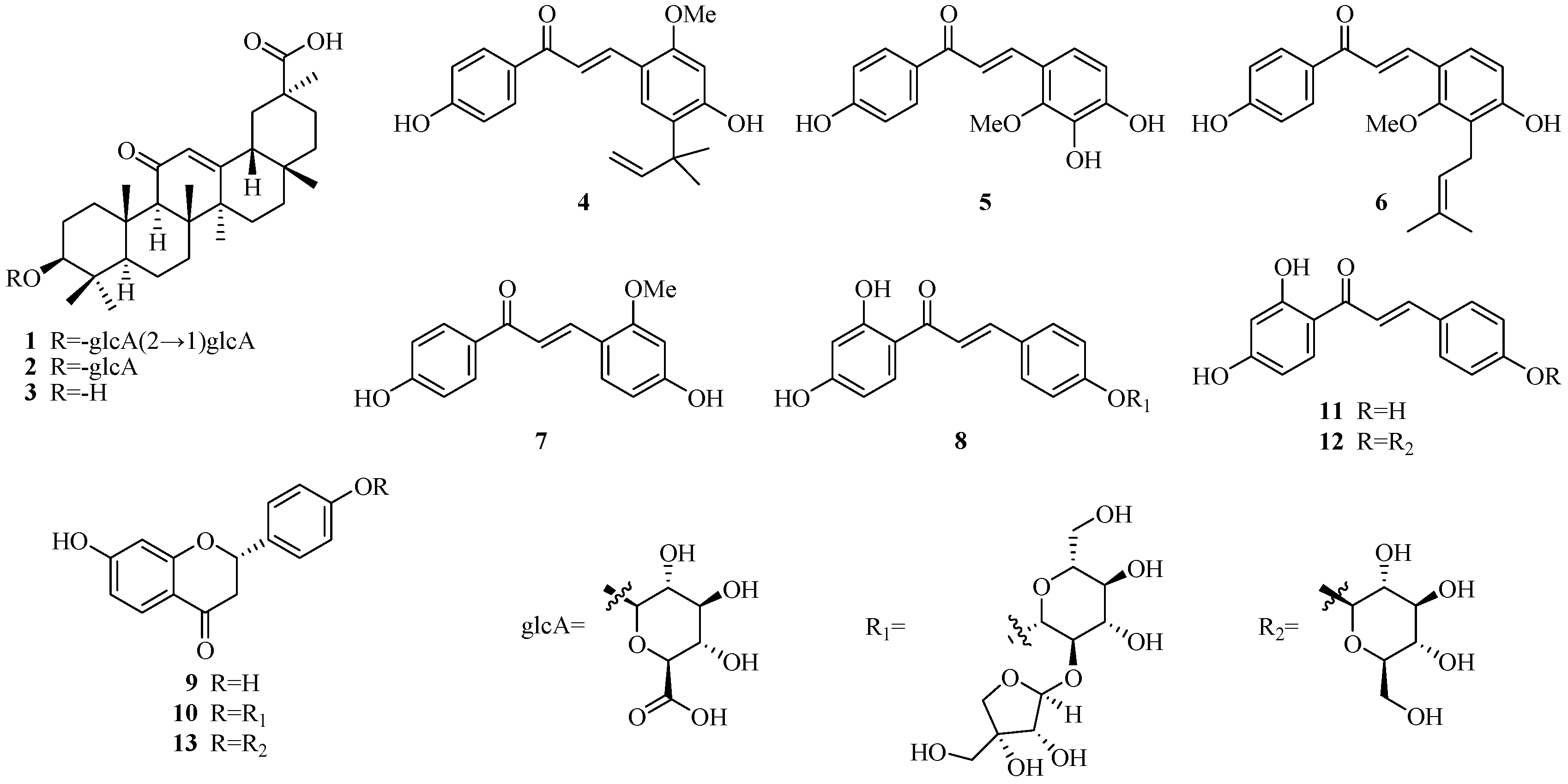
3.1. Assayed Compounds
3.2. Chemicals and Reagents
3.3. Instrumental Analyses
3.4. Cell Culture
3.5. Caco-2 Cell Permeability
3.6. Time- and Concentration-Dependent Transport of the Compounds 2–7 and 9 across the Caco-2 Cell Monolayer
3.7. Intracellular Accumulation and Recovery
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2015; Volume I, pp. 86–87. ISBN 978-7-5067-7337-9. [Google Scholar]
- Zhang, Q.Y.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Song, W.; Ji, S.; Wang, Q.; Guo, D.A.; Ye, M. Separation and characterization of phenolic compounds and triterpenoid saponins in licorice (Glycyrrhiza. uralensis) using mobile phase-dependent reversed-phase × reversed-phase comprehensive two-dimensional liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2015, 1402, 36–45. [Google Scholar] [PubMed]
- Karkanis, A.; Martins, N.; Petropoulos, S.A.; Ferreira, I.C.F.R. Phytochemical composition, health effects, and crop management of liquorice (Glycyrrhiza. glabra L.): Α medicinal plant. Food Rev. Int. 2016, 1–22. [Google Scholar] [CrossRef]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza. glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef]
- Adianti, M.; Aoki, C.; Komoto, M.; Deng, L.; Shoji, I.; Wahyuni, T.S.; Lusida, M.I.; Soetjipto, H.; Fuchino, N.; Hotta Kawahara, H. Anti-hepatitis C virus compounds obtained from Glycyrrhiza. uralensis and other Glycyrrhiza. species. Microbiol. Immunol. 2014, 58, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Res. 2009, 83, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as antiviral agent against hepatitis C virus. J. Transl. Med. 2011, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Sakamoto, M.; Matsushita, M.; Fujita, T.; Nishioka, K. Glycyrrhizin inhibits the lytic pathway of complement—possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Micrrobiol. Immunol. 2000, 44, 799–804. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Ci, X.X.; Wei, M.M.; Yang, X.F.; Cao, Q.J.; Guan, M.F.; Li, H.Y.; Deng, Y.H.; Feng, H.H.; Deng, X.M. Licochalcone A inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Eom, T.K.; Jeong, Y.H. Glycyrrhiza. glabra L. extract inhibits LPS-induced inflammation in RAW macrophages. J. Nutr. Sci. Vitaminol. 2015, 61, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Zhao, T.; Feng, M.; Chen, H.; Zhuang, M.; Lin, L. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza. glabra L. leaf and root extracts. Molecules 2014, 19, 9101–9113. [Google Scholar] [CrossRef] [PubMed]
- Vaya, J.; Belinky, P.A.; Aviram, M. Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic. Biol. Med. 1997, 23, 302–313. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Wei, J.H.; Fang, S.Q.; Tang, Y.P.; Cheng, H.B.; Wang, T.L.; Li, C.Y.; Peng, G.P. Hepatoprotective triterpene saponins from the roots of Glycyrrhiza. inflata. Molecules 2015, 20, 6273–6283. [Google Scholar] [CrossRef] [PubMed]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates metabolic syndrome-induced liver damage in experimental rat model. Mol. Cell. Biochem. 2015, 409, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Christensen, S.B.; Blom, J.; Lemmich, E.; Nadelmann, L.; Fich, K.; Theander, T.G.; Kharazmi, A. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother. 1993, 37, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D.; et al. Antimicrobial potential of Glycyrrhiza. glabra roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Gupta, V.K.; Luqman, S.; Negi, A.S.; Kumar, J.K.; Shanker, K.; Saikia, D.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P.S. Antifungal activity of Glycyrrhiza. glabra extracts and its active constituent glabridin. Phytother. Res. 2009, 23, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Asha, M.K.; Debraj, D.; Prashanth, D.S.; Edwin, J.R.; Srikanth, H.S.; Muruganantham, N.; Dethe, S.M.; Anirban, B.; Jaya, B.; Deepak, M.; et al. In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J. Ethnopharmacol. 2013, 145, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.S.; Prakash, O.; Pal, A.; Khan, F.; Bawankule, D.U.; Darokar, M.P. Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum. Parasitol. Int. 2014, 63, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Chakotiya, A.S.; Tanwar, A.; Narula, A.; Sharma, R.K. Alternative to antibiotics against Pseudomonas aeruginosa: Effects of Glycyrrhiza. glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time-kill activity. Microb. Pathog. 2016, 98, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Smolarczyk, R.; Cichoń, T.; Matuszczak, S.; Mitrus, I.; Lesiak, M.; Kobusińska, M.; Kamysz, W.; Jarosz, M.; Sieroń, A.; Szala, S. The role of glycyrrhizin, an inhibitor of HMGB1 protein, in anticancer therapy. Arch. Immunol. Ther. Exp. 2012, 60, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Park, S.Y.; Kwon, G.T.; Lee, K.W.; Kang, Y.H.; Choi, M.S.; Yun, J.W.; Jeon, J.H.; Jun, J.G.; Park, J.H.Y. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev. Res. 2013, 6, 603–613. [Google Scholar] [CrossRef] [PubMed]
- He, S.Q.; Gao, M.; Fu, Y.F.; Zhang, Y.N. Glycyrrhizic acid inhibits leukemia cell growth and migration via blocking AKT/mTOR/STAT3 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 5175–5181. [Google Scholar] [PubMed]
- Kamei, J.; Nakamura, R.; Ichiki, H.; Kubo, M. Antitussive principles of Glycyrrhizae radix, a main component of the Kampo preparations Bakumondo-to (Mai-men-dong-tang). Eur. J. Pharmacol. 2003, 469, 159–163. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.W.; Bae, E.A.; Lee, B.; Seung, H.L.; Jeong, A.K.; Kim, Y.S.; Kim, D.H. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Med 2007, 73, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Ji, S.; Yu, S.W.; Lin, X.H.; Jin, H.W.; Duan, Y.K.; Zhang, L.R.; Guo, D.A.; Ye, M. Identification of key licorice constituents which interact with cytochrome P450: Evaluation by LC/MS/MS cocktail assay and metabolic profiling. AAPS J. 2014, 16, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Yang, X.D.; Wang, Y.; Ma, L.; Zhang, Y.; Yang, X.G.; Wang, K. Establishment of Caco-2 cell monolayer model and standard operation procedure for assessing intestinal absorption of chemical components of traditional Chinese medicine. J. Chin. Integr. Med. 2007, 5, 634–641. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Guidance for Industry, Bioanalytical Method Validation. Available online: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm368107.pdf. (accessed on 27 September 2017).
- Yang, X.W. Absorption, Distribution, Metabolism, Excretion, Toxicity and Activity in Traditional Chinese Medicines, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2006; pp. 1075–1104. ISBN 7-5067-3337-4. [Google Scholar]
- Tian, X.J.; Yang, X.W.; Yang, X.D.; Wang, K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009, 367, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Huang, Y.D.; Yang, X.W. Intestinal permeability of liquiritin and isoliquiritin in the Caco-2 cell monolayer model. J. Chin. Pharm. Sci. 2010, 19, 451–458. [Google Scholar] [CrossRef]
- Chan, O.H.; Stewart, B.H. Physicochemical and drug-delivery considerations for oral drug bioavailability. Drug Discov. Today 1996, 1, 461–473. [Google Scholar] [CrossRef]
- Wu, X.W.; Wei, W.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Yang, Y.F.; Zhong, G.Y.; Liu, H.N.; Yang, S.L. Anti-inflammatory phenolic acid esters from the roots and rhizomes of Notopterygium. incisium and their permeability in the human Caco-2 monolayer cell model. Molecules 2017, 22, 935. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W. Discovery strategy for effective and active constituents of Chinese material medica based on the processes of metabolism and disposition in intra-body. China J. Chin. Mater. Med. 2007, 32, 365–370. [Google Scholar]
- Yang, X.W. Substance basis research on Chinese materia medica is one of key scientific problems of inheriting, development and innovation of Chinese materia medica. China J. Chin. Mater. Med. 2015, 40, 3429–3434. [Google Scholar]
Sample Availability: Samples of the compounds 1–10 are not available from the authors. |


| Analytes | Papp AP→BL b (×10−6 cm/s) | Papp BL→AP c (×10−6 cm/s) | Efflux Ratio d | MW | Log D (pH = 7.35) |
|---|---|---|---|---|---|
| 1 | 0 | 0 | – | 822 | −8.02 |
| 2 | 1.55 ± 0.09 | 1.65 ± 0.95 | 1.06 | 646 | −2.94 |
| 3 | 12.63 ± 0.40 | 14.00 ± 1.30 | 1.11 | 470 | 2.57 |
| 4 | 1.68 ± 0.13 | 1.78 ± 0.03 | 1.06 | 338 | 4.48 |
| 5 | 24.71 ± 2.80 | 30.18 ± 2.40 | 1.22 | 286 | 2.91 |
| 6 | 22.51 ± 1.00 | 26.44 ± 1.30 | 1.17 | 338 | 4.62 |
| 7 | 20.70 ± 1.00 | 23.80 ± 0.30 | 1.15 | 270 | 3.43 |
| 8 | 0 | 0 | – | 550 | −0.94 |
| 9 | 17.80 ± 0.60 | 15.68 ± 0.30 | 0.88 | 256 | 2.95 |
| 10 | 0 | 0 | – | 550 | −0.23 |
| 11 | 14.60 ± 0.10 | 9.35 ± 0.43 | 0.64 | 256 | 2.50 |
| 12 | 0.87 ± 0.02 | 0.77 ± 0.07 | 0.89 | 418 | −0.48 |
| 13 | 0.54 ± 0.02 | 0.53 ± 0.03 | 0.98 | 418 | 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-X.; Liu, G.-Y.; Yang, Y.-F.; Wu, X.-W.; Xu, W.; Yang, X.-W. Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 1627. https://doi.org/10.3390/molecules22101627
Wang X-X, Liu G-Y, Yang Y-F, Wu X-W, Xu W, Yang X-W. Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model. Molecules. 2017; 22(10):1627. https://doi.org/10.3390/molecules22101627
Chicago/Turabian StyleWang, Xiao-Xue, Gui-Yan Liu, Yan-Fang Yang, Xiu-Wen Wu, Wei Xu, and Xiu-Wei Yang. 2017. "Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model" Molecules 22, no. 10: 1627. https://doi.org/10.3390/molecules22101627




