Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Micro-Potassium Permanganate Method
2.3. Micro-Iodometric Method
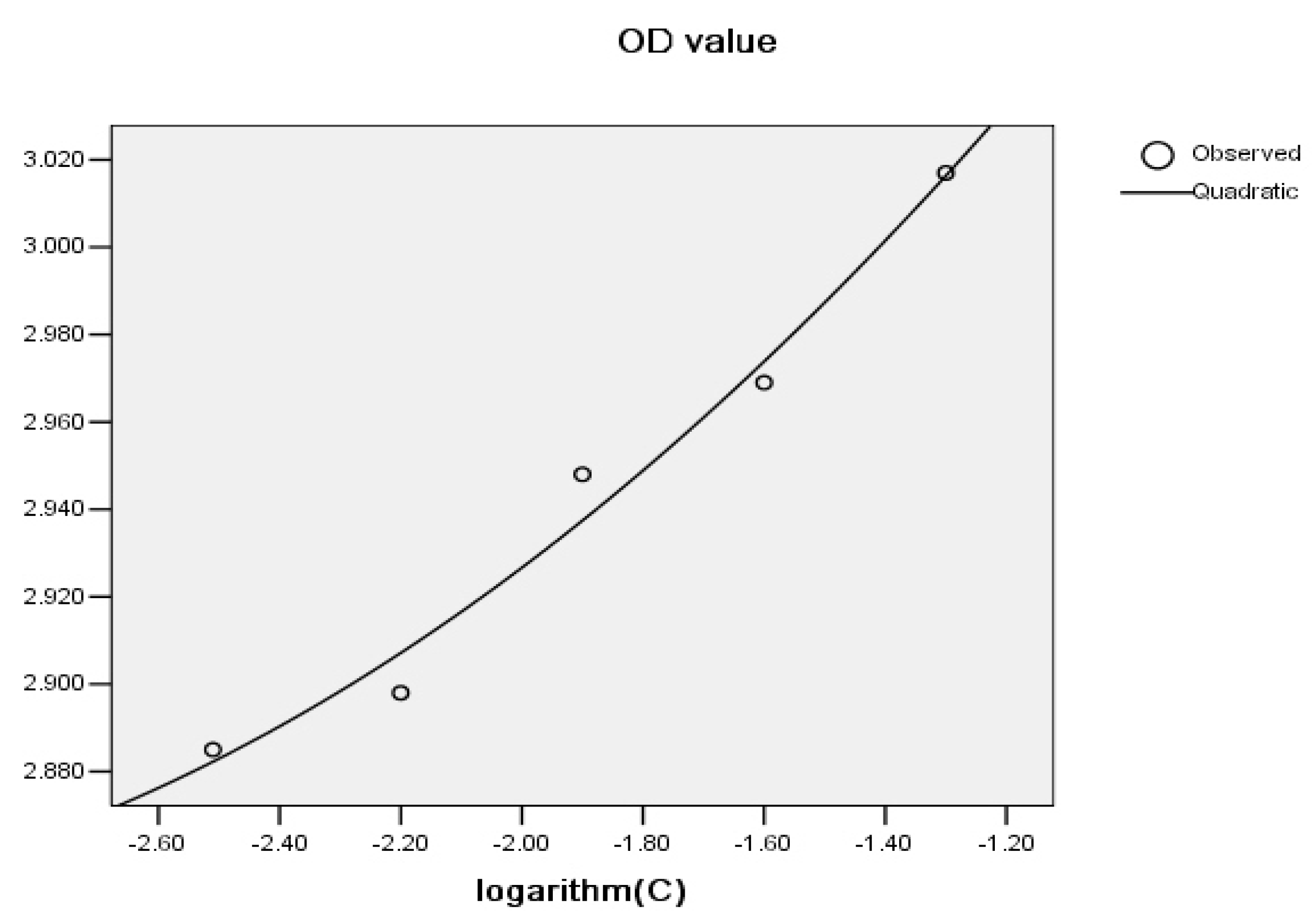
2.4. Quantitative Analysis
2.5. Statistical Analysis
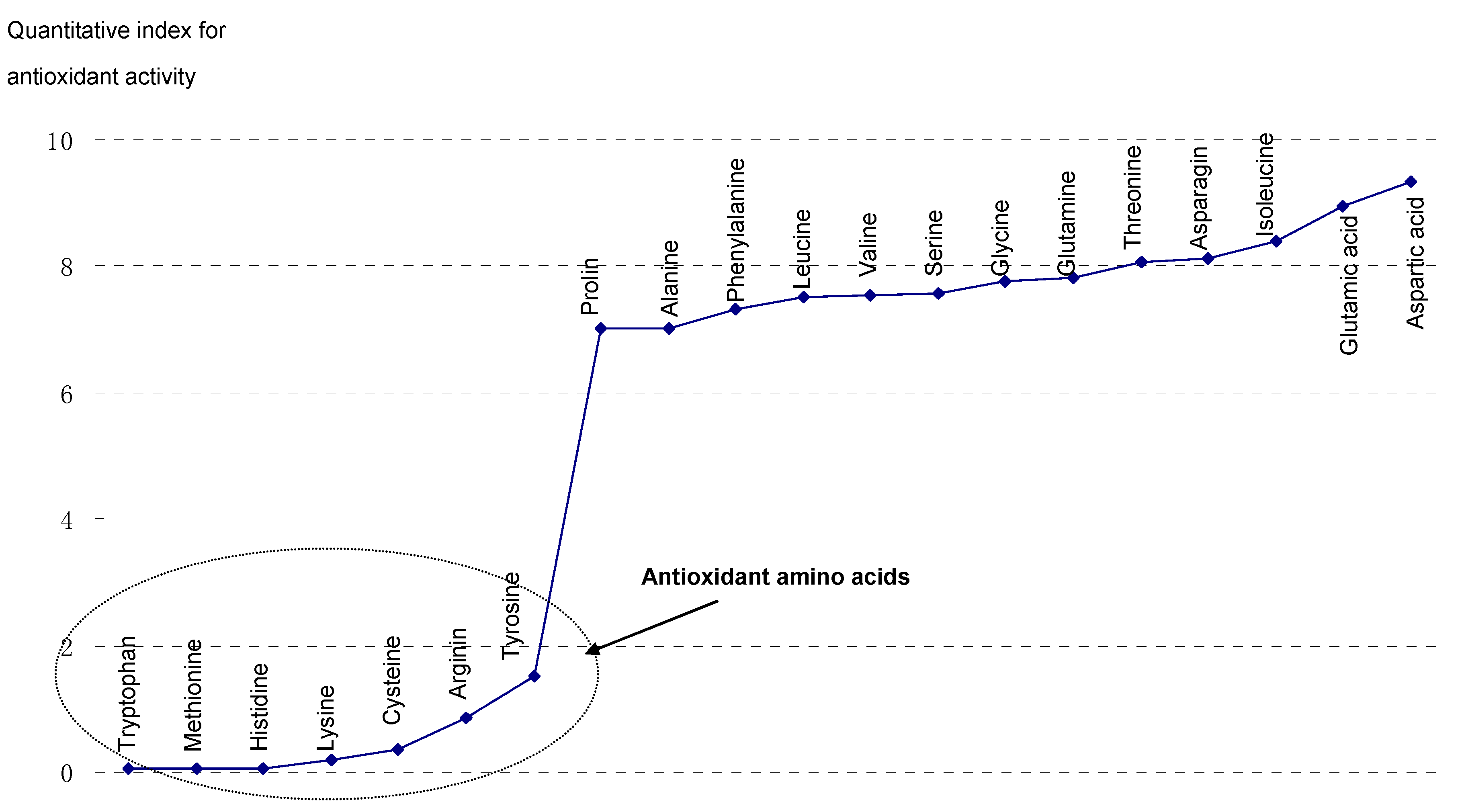
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflict of Interest
References
- Luangwattananun, P.; Eiamphungporn, W.; Songtawee, N.; Bülow, L.; Isarankura, N.; Ayudhya, C.I.N.; Prachayasittikul, V.; Yainoy, S. Improving enzymatic activities and thermostability of a tri-functional enzyme with SOD, catalase and cell-permeable activities. J. Biotechnol. 2017, 247, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Michał, S.; Monika, M.; Małgorzata, P.; Rusłan, S.; Grytner-Zięcina, B. Enzymatic antioxidant system in the cestode Hymenolepis diminuta after chronic infection of the rat. Cent. Eur. J. Biol. 2012, 7, 987–995. [Google Scholar]
- Pieme, C.A.; Tatangmo, J.A.; Simo, G.; Nya, P.C.B.; Moor, V.J.A.; Moukette, B.M.; Nzufo, F.T.; Nono, B.L.N.; Sobngwi, E. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res. Notes 2017, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Serbanescu, G.L.; Gruia, M.I.; Bara, M.; Anghel, R.M. The evaluation of the oxidative stress for patients receiving neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J. Med. Life 2017, 10, 99–103. [Google Scholar] [PubMed]
- Pietro, V.; Mercedes, A.P.; Carme, R. Catalases versus peroxidases: DFT investigation of H2O2 oxidation in models systems and implications for heme protein engineering. J. Inorg. Biochem. 2012, 117, 292–297. [Google Scholar]
- Italia, K.; Chandrakala, S.; Ghosh, K.; Colah, R. Can hydroxyurea serve as a free radical scavenger and reduce iron overload in β-thalassemia patients? Free Radic. Res. 2016, 50, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017, 9, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, S.; Lasekan, O.; Tan, C.P.; Abas, F.; Wei, L.S. Comparative study of the antioxidant activities of some lipase-catalyzed alkyl dihydrocaffeates synthesized in ionic liquid. Food Chem. 2017, 224, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Barim-Oz, O.; Sahin, H. The influence of dietary antioxidant on ovarian eggs and levels of vitamin E, C, A, astaxanthin, β-carotene and oxidative stres in tissues of Astacus leptodactylus (Eschscholtz) during reproduction. Cell. Mol. Biol. (Noisy le Grand) 2016, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Montani, M.S.G.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-Derived Compounds: A Basis for the Creation of New Pharmacological Agents for Cancer Prevention and Therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, Y.B.; Aydin, C.; Gorgisen, G. The effects of melatonin on oxidative stress and prevention of primordial follicle loss via activation of mTOR pathway in the rat ovary. Cell. Mol. Biol. (Noisy le Grand) 2017, 63, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dattilo, M.; Macut, D.; Duntas, L.; Gonos, E.S.; Goulis, D.G.; Gantenbein, C.K.; Kapetanou, M.; Koukkou, E.; Lambrinoudaki, I.; et al. Combo endo team: 2016. Mechanisms in endocrinology: Aging and anti-aging: A Combo-Endocrinology overview. Eur. J. Endocrinol. 2017, 176, R283–R308. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; He, Y.; Zheng, Q.; Wang, M.; Wu, Y.; Zhang, Y.; Wang, C. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur. J. Pharmacol. 2017, 797, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Poth, A.G.; Yendo, A.C.; Fett-Neto, A.G.; Craik, D.J. Isolation and Characterization of Cyclotides from Brazilian Psychotria: Significance in Plant Defense and Co-occurrence with Antioxidant Alkaloids. J. Nat. Prod. 2016, 79, 3006–3013. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Kumar, B.D.; Hemalathab, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Winkler-Moser, J.K. Antioxidant activity of amino acids in soybean oil at frying temperature: Structural effects and synergism with tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Huichun, W.; Huaming, C.; Chyuanyuan, S. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar]
- Zhang, M.; Liu, N.; Liu, H. Determination of the Total Mass of Antioxidant Substances and Antioxidant Capacity per Unit Mass in Serum Using Redox Titration. Bioinorg. Chem. Appl. 2014, 2014, 928595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, J.; Wang, Z.; Liu, H. Evaluating the Risk of Tumors Diseases Based on Measurement of Urinary and Serumal Antioxidants Using the New Agar Diffusion Methods. Oxid. Med. Cell. Longev. 2017, 2017, 6578453. [Google Scholar] [CrossRef] [PubMed]
- Marian, V.; Dieter, L.; Jan, M.; Mark, T.D.C.; Milan, M.; Joshua, T. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar]
- Cao, T.; Xu, N.; Wang, Z.; Liu, H. Effects of Glutathione S-Transferase Gene Polymorphisms and Antioxidant Capacity per Unit Albumin on the Pathogenesis of Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell. Longev. 2017, 2017, 6232397. [Google Scholar] [CrossRef] [PubMed]
- Kanti, B.P.; Mohd, M.M.; Pawan, K.M.; Syed, I.R. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis. Mark. 2010, 29, 31–36. [Google Scholar]
- Stryer, L. Biochemistry, 4th ed.; W H Freeman and Company: New York, NY, USA, 1995; pp. 19–23. [Google Scholar]
- Garrett, A.R.; Weagel, E.G.; Martinez, A.D.; Heaton, M.; Robison, R.A.; O’Neill, K.L. A novel method for predicting antioxidant activity based on amino acid structure. Food Chem. 2014, 158, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, M.; Liu, H. Total Antioxidant Capacity of Serum Determined Using the Potassium Permanganate Agar Method Based on Serum Diffusion in Agar. Bioinorg. Chem. Appl. 2015, 2015, 406071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, N.; Zhang, M.; Liu, H. A New Method for Measuring Total Antioxidant Capacity in Urine using the Iodine Starch agar Based on agar Diffusion. Curr. Anal. Chem. 2016, 11, 1–6. [Google Scholar] [CrossRef]
- Cao, T.; He, M.; Bai, T.; Liu, H. Establishment of a Method for Measuring Antioxidant Capacity in Urine, Based on Oxidation Reduction Potential and Redox Couple I2/KI. Bioinorg. Chem. Appl. 2016, 2016, 7054049. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
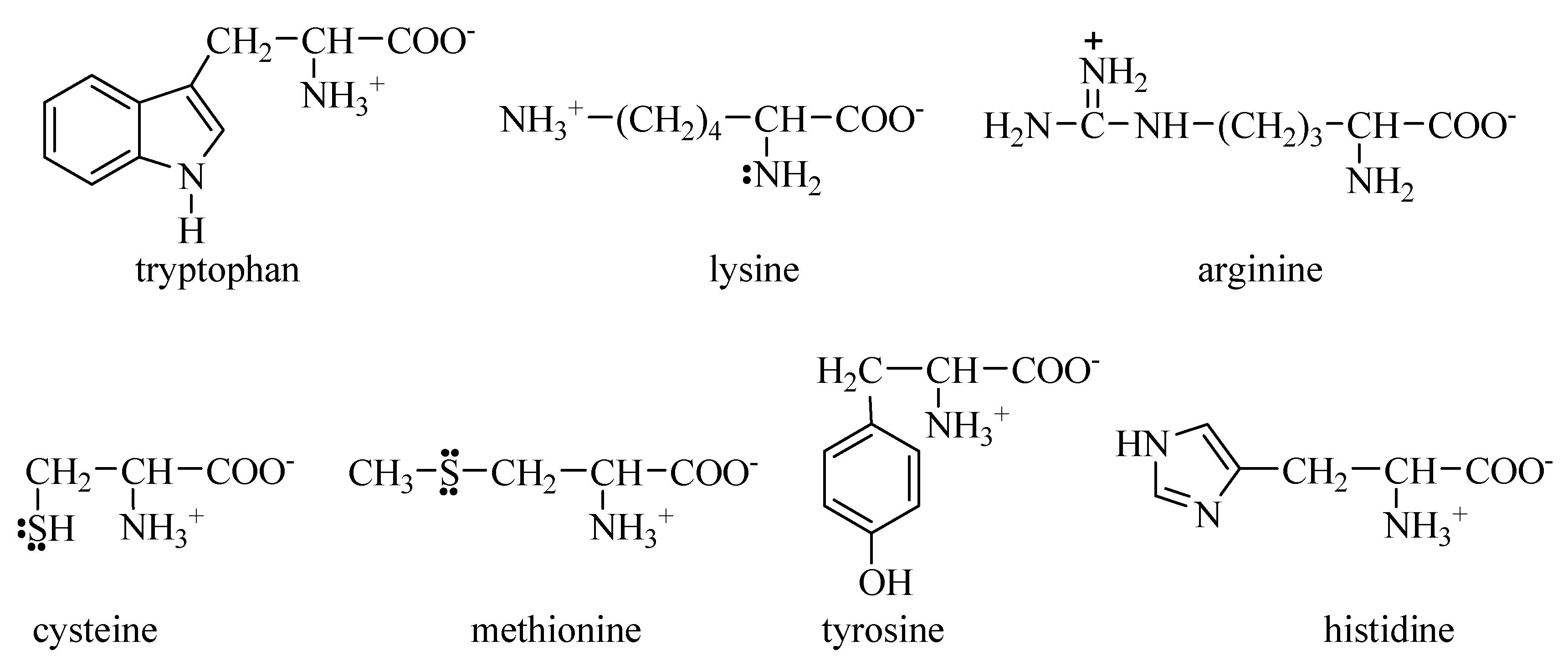
| Amino Acid | Molecular Weight | Amino Acid | Molecular Weight | Amino Acid | Molecular Weight |
|---|---|---|---|---|---|
| Alanine | 89.09 | Glycine | 75.07 | Proline | 115.13 |
| Arginine | 174.20 | Histidine | 155.15 | Serine | 105.09 |
| Asparagine | 132.12 | Phenylalanine | 165.19 | Threonine | 119.12 |
| Aspartic acid | 133.10 | Isoleucine | 131.17 | Tryptophan | 204.23 |
| Cysteine | 121.16 | Leucine | 131.17 | Tyrosine | 181.19 |
| Valine | 117.15 | Lysine | 146.19 | Glutamine | 146.14 |
| Glutamic acid | 147.13 | Methionine | 149.21 | - | - |
| Amino Acid | Antioxidant Activity | Amino Acid | Antioxidant Activity | ||||
|---|---|---|---|---|---|---|---|
| AUC-Mn | AUC-I | Total | AUC-Mn | AUC-I | Total | ||
| Tryptophan | 0.953 | 0.057 | 0.054 | Valine | 3.551 | 2.121 | 7.532 |
| Methionine | 1.151 | 0.049 | 0.056 | Serine | 3.397 | 2.232 | 7.582 |
| Histidine | 1.198 | 0.056 | 0.067 | Glycine | 3.572 | 2.177 | 7.776 |
| Lysine | 1.931 | 0.158 | 0.305 | Glutamine | 3.556 | 2.198 | 7.816 |
| Cysteine | 1.851 | 0.198 | 0.366 | Threonine | 3.507 | 2.297 | 8.056 |
| Arginine | 2.969 | 0.289 | 0.858 | Asparagine | 3.598 | 2.257 | 8.121 |
| Tyrosine | 1.146 | 1.324 | 1.517 | Isoleucine | 3.596 | 2.334 | 8.393 |
| Proline | 3.202 | 2.188 | 7.006 | Glutamic | 3.584 | 2.498 | 8.953 |
| Alanine | 3.558 | 1.972 | 7.016 | Aspartic | 3.773 | 2.475 | 9.338 |
| Phenylalanine | 3.471 | 2.107 | 7.313 | r (p) | 0.869 (<0.001) | ||
| Leucine | 3.530 | 2.125 | 7.501 | Blank control | 3.610 | 2.381 | 8.595 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. https://doi.org/10.3390/molecules22122066
Xu N, Chen G, Liu H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules. 2017; 22(12):2066. https://doi.org/10.3390/molecules22122066
Chicago/Turabian StyleXu, Naijin, Guanqun Chen, and Hui Liu. 2017. "Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation" Molecules 22, no. 12: 2066. https://doi.org/10.3390/molecules22122066





