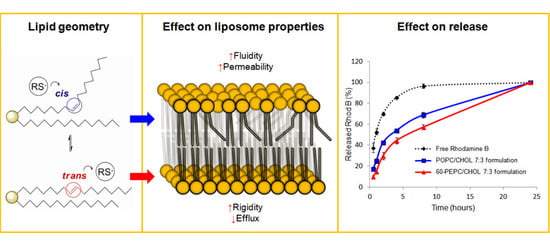
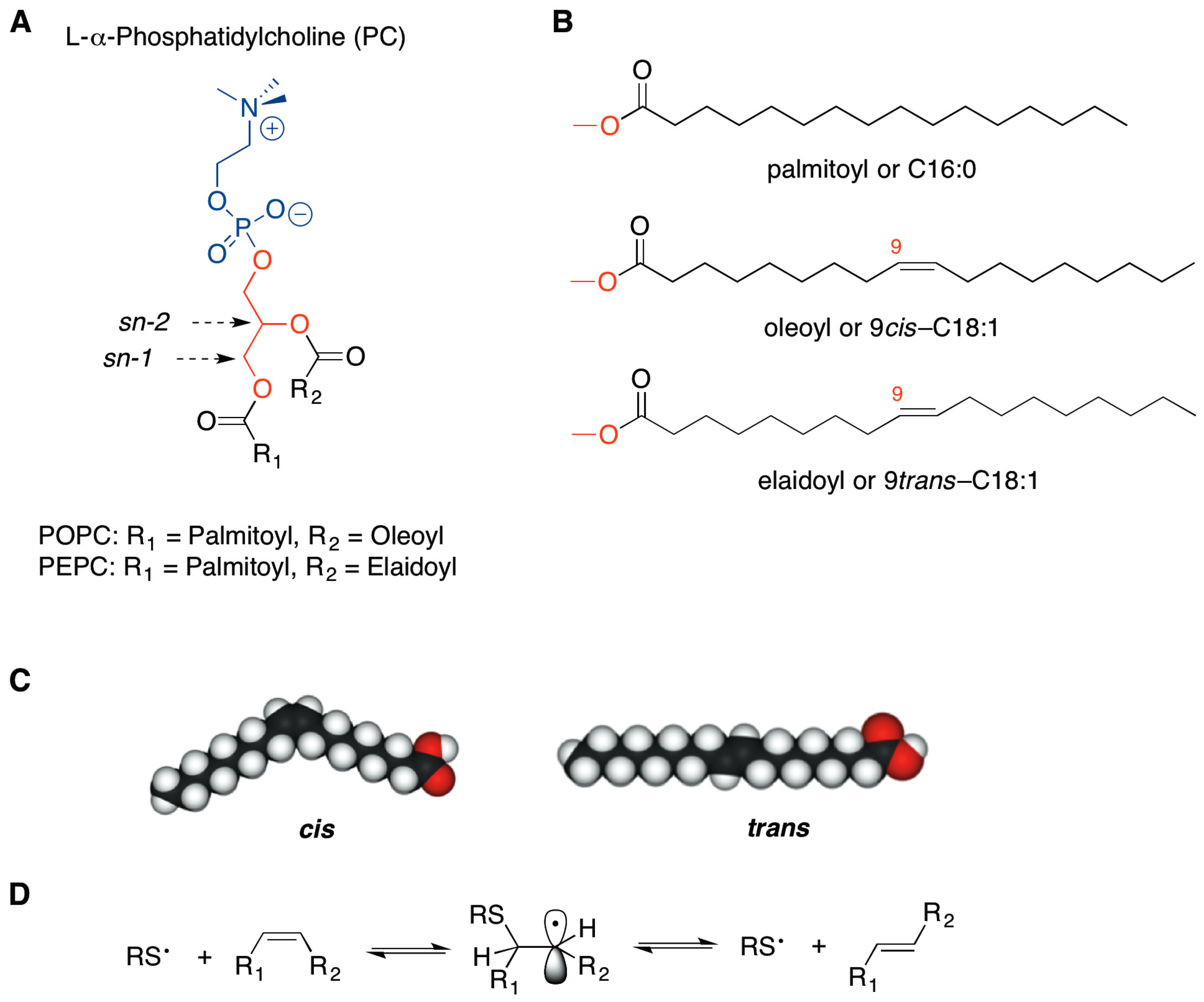
trans-Double Bond-Containing Liposomes as Potential Carriers for Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
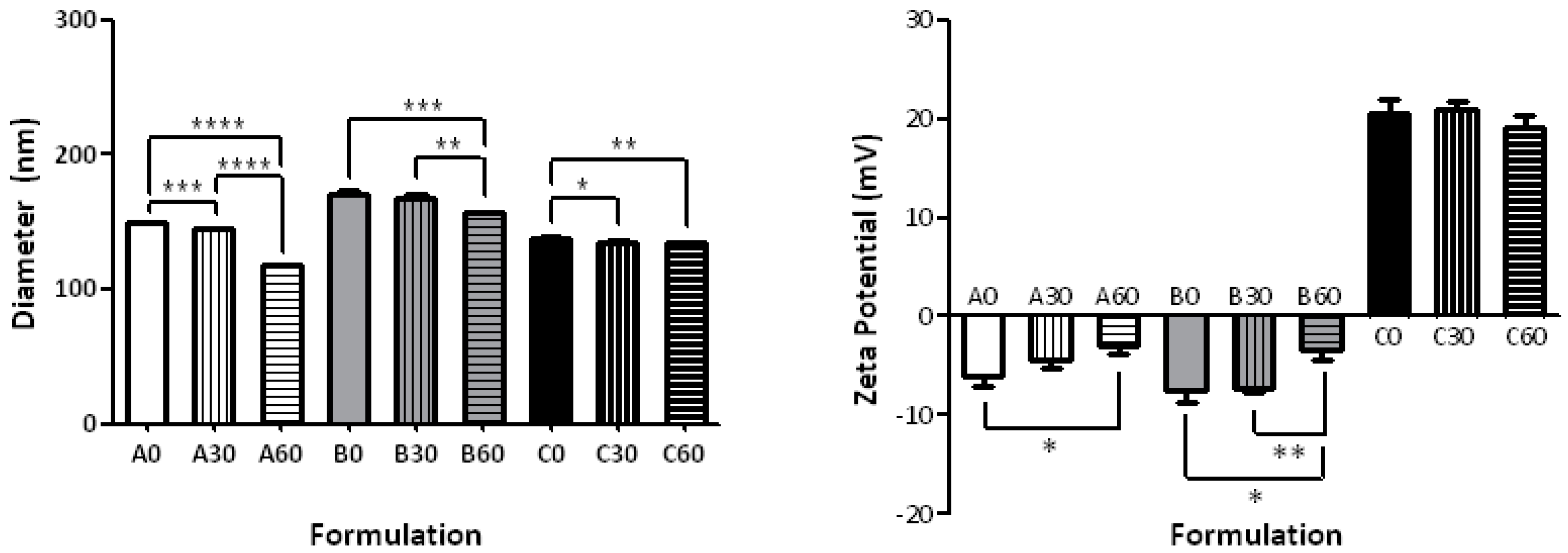
2.1. Effect of the Formulation on Size and ζ-Potential
2.2. Effect of the Formulation on Stability (Size vs. Time)
2.3. Atomic Force Microscopy
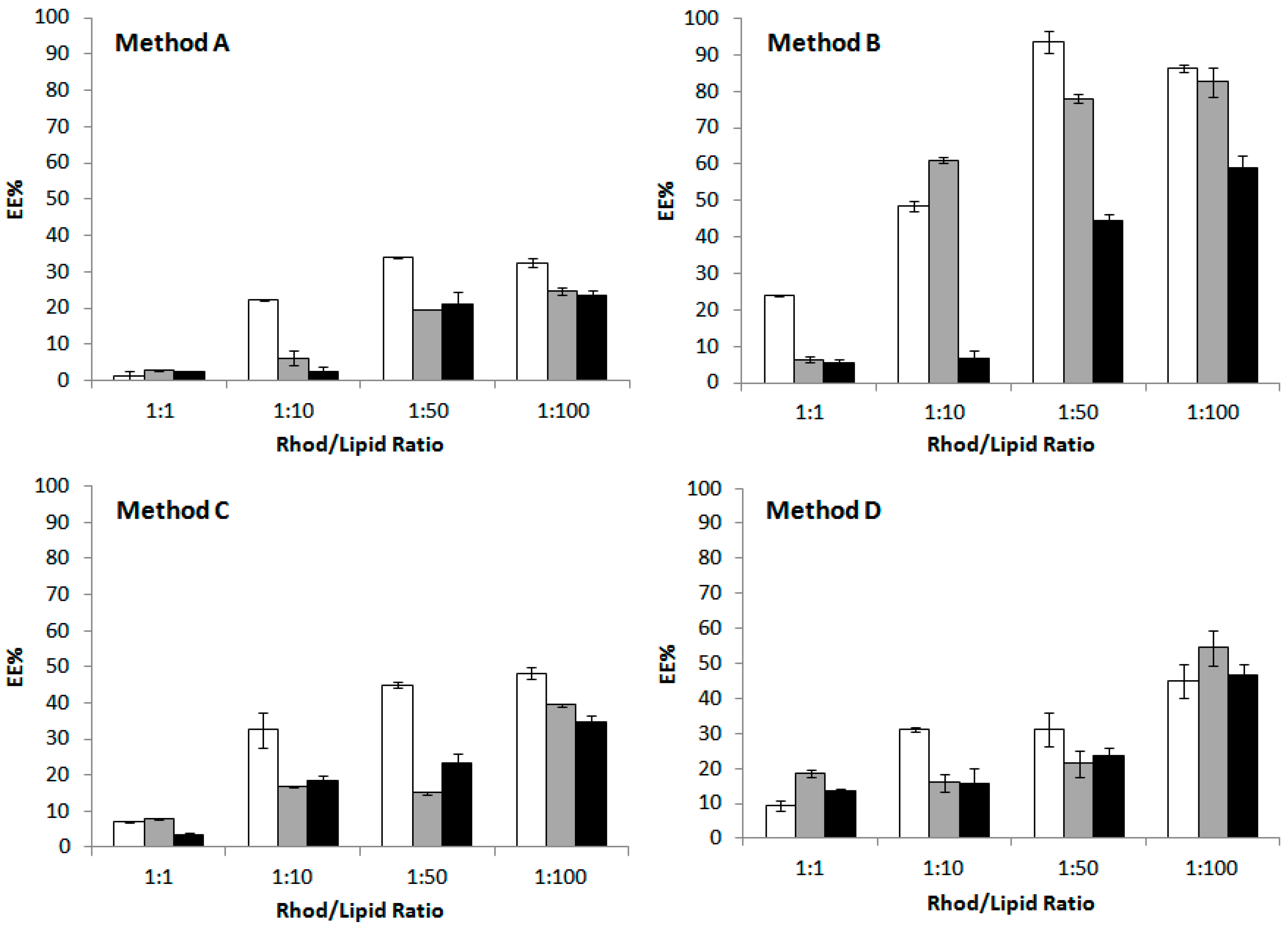
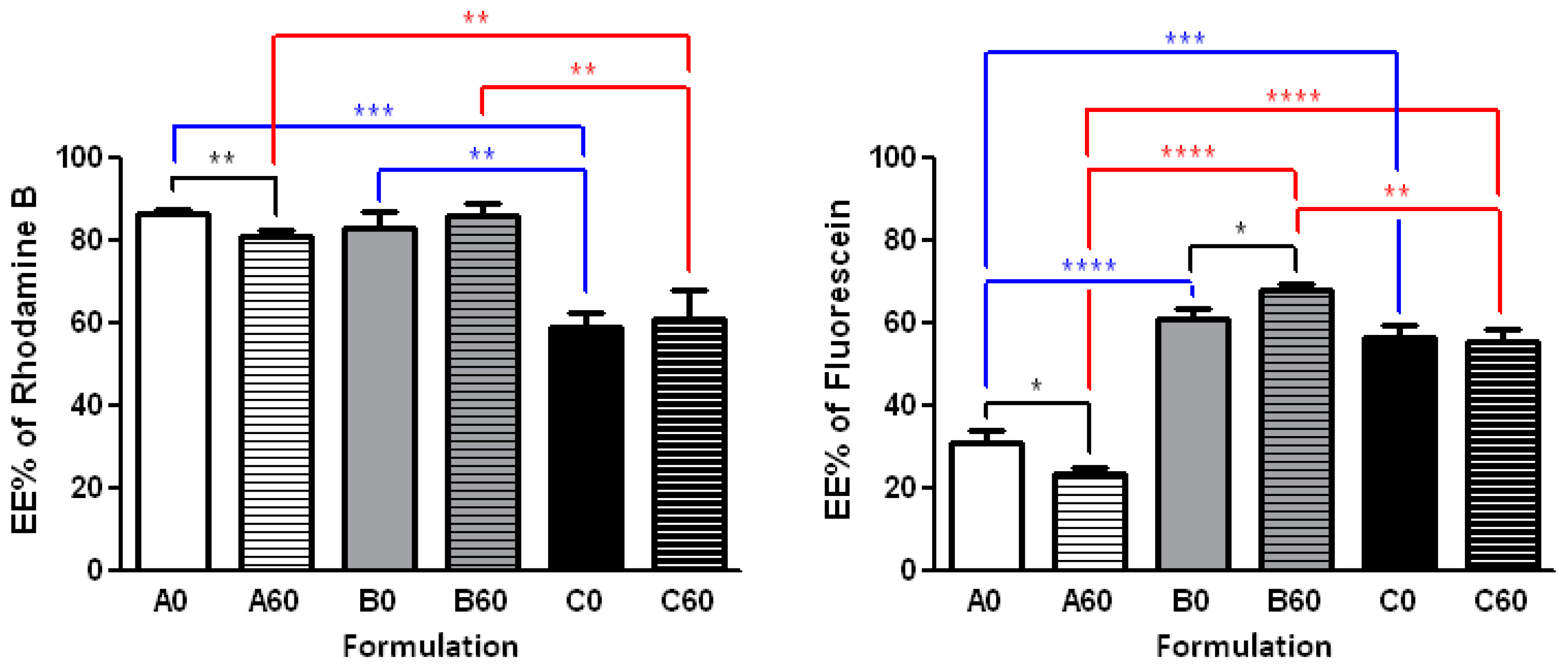
2.4. Effect of the Procedure on the Encapsulation Efficiency
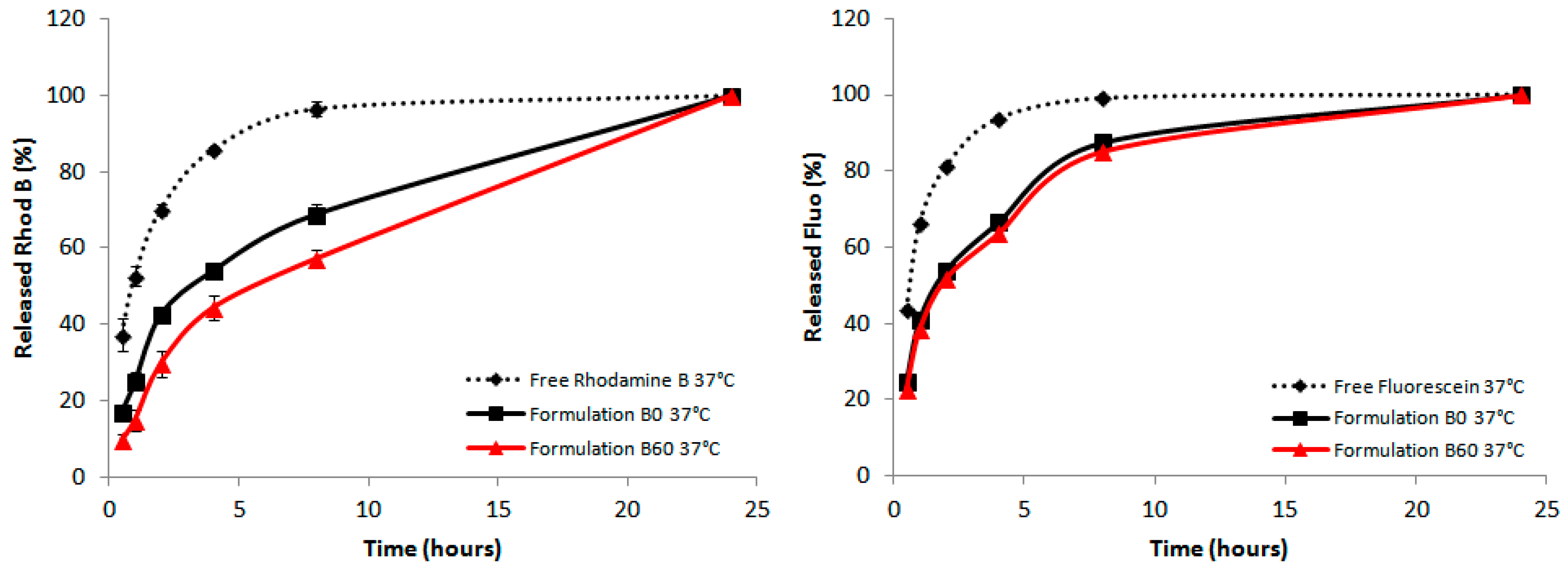
2.5. Effect of the Liposomal Formulation on the Release
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Synthesis and Purification of PEPC
3.2.2. Liposome Preparation
3.2.3. Dye Encapsulation
3.2.4. Encapsulation Efficiency (EE%)
3.2.5. In Vitro Release
3.2.6. Lipid Extraction and GC Analysis
3.2.7. Dynamic Light Scattering (DLS)
3.2.8. AFM Analyses
3.2.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| CHOL | cholesterol |
| D/L | dye to lipid molar ratio |
| DLS | dynamic light scattering |
| DPPC | 1,2-dipalmitoylphosphatidylcholine |
| EE | encapsulation efficiency |
| FAME | fatty acid methyl ester |
| GC | gas chromatography |
| LUVET | large unilamellar vesicle by extrusion technique |
| MLV | multilamellar vesicle |
| MTMAB | myristyl trimethyl ammonium bromide |
| PBS | phosphate buffered saline |
| PDI | polydispersity index |
| PEPC | 1-palmitoyl-2-elaidoylphosphatidylcholine |
| POPC | 1-palmitoyl-2-oleoylphosphatidylcholine |
| POPE | 1-palmitoyl-2-oleoylphosphatidylethanolamine |
| TFA | trans fatty acid |
| TLC | thin layer chromatography |
References
- Ferreri, C.; Chatgilialoglu, C. Membrane lipidomics and the geometry of unsaturated fatty acids, from biomimetic models to biological consequences. In Lipidomics: Methods and Protocols; Armstrong, D., Ed.; Humana Press: New York, NY, USA, 2009; Volume 1, pp. 391–411. ISBN 978–1-60761-322-0. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C. Trans lipids: The free radical path. Acc. Chem. Res. 2005, 38, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Geometrical trans lipid isomers: A new target for lipidomics. ChemBioChem 2005, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, H.; Okajima, N.; Sasaki, S.; Higashi, S.; Murata, N. The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochim. Biophys. Acta 1991, 1084, 13–20. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Sébédio, J.L.; Christie, W.W. Metabolism of trans fatty acid isomers. In Trans Fatty Acids in Human Nutrition, 2nd ed.; Destaillats, F., Sébédio, J.L., Dionisi, F., Chardigny, J.M., Eds.; Oily Press: Bridgwater, UK, 2009; pp. 163–194. ISBN 9780955251238. [Google Scholar]
- Fournier, V.; Destaillats, F.; Juanéda, P.; Dionisi, F.; Lambelet, P.; Sébédio, J.L.; Berdeaux, O. Thermal degradation of long-chain polyunsaturated fatty acids during deodorization of fish oil. Eur. J. Lipid Sci. Technol. 2006, 108, 33–42. [Google Scholar] [CrossRef]
- Ferreri, C.; Costantino, C.; Chatgilialoglu, C.; Landi, L.; Mulazzani, Q.G. The thiyl radical-mediated isomerization of cis-monounsaturated fatty acid residues in phospholipids: A novel path of membrane damage? Chem. Commun. 1999, 407–408. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Ballestri, M.; Mulazzani, Q.G.; Landi, L. Cis-trans-isomerization of monounsaturated fatty acid residues in phospholipids by thiyl radicals. J. Am. Chem. Soc. 2000, 122, 4593–4601. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Sansone, A.; Barata-Vallejo, S.; Chatgilialoglu, C.; Ferreri, C. Bleomycin-induced trans lipid formation in cell membranes and in liposome models. Org. Biomol. Chem. 2015, 13, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Stender, S.; Szecsi, P.B.; Pedersen, S.B.; Basu, S.; Hellgren, L.I.; Newman, J.W.; Larsen, T.M.; Haugaard, S.B.; Astrup, A. Effect of industrially produced trans fat on markers of systemic inflammation: Evidence from a randomized trial in women. J. Lipid Res. 2011, 52, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Pierotti, S.; Barbieri, A.; Zambonin, L.; Landi, L.; Rasl, S.; Luisi, P.L.; Barigelletti, F.; Chatgilialoglu, C. Comparison of phosphatidylcholine vesicle properties related to geometrical isomerism. Photochem. Photobiol. 2006, 82, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Pierotti, S.; Chatgilialoglu, C.; Barbieri, A.; Barigelletti, F. Probing the influence of cis-trans isomers on model lipid membrane fluidity using cis-parinaric acid and a stop-flow technique. Chem. Commun. 2006, 5, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Berkland, C.; Kipper, M.J.; Narasimhan, B.; Kim, K.K.; Pack, D.W. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J. Control. Release 2004, 94, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Neofytou, E.; Cahill, T.J.; Beygui, R.E.; Zare, R.N. Drug Release from Electric-Field-Responsive Nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Igal, A.R. Roles of stearoylCoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers 2011, 3, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; de Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; Haldar, S. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain-backbone linkage. Biochim. Biophys. Acta Biomembr. 2000, 1467, 39–53. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, K.-E.; Kim, J.-J.; Lim, S.-H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 5005. [Google Scholar] [CrossRef] [PubMed]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar] [CrossRef]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Seddon, J.M.; Cevc, G.; Marsh, D. Calorimetric studies of the gel-fluid (L.beta.-L.alpha.) and lamellar-inverted hexagonal (L.alpha.-HII) phase transitions in dialkyl-and diacylphosphatidylethanolamines. Biochemistry 1983, 22, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Z.; Vail, W.J.; Szoka, F.C. Acid-and calcium-induced structural changes in phosphatidylethanolamine membranes stabilized by cholesteryl hemisuccinate. Biochemistry 1985, 24, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Pinnaduwage, P.; Schmitt, L.; Huang, L. Use of a quaternary ammonium detergent in liposome mediated DNA transfection of mouse L-cells. Biochim. Biophys. Acta 1989, 985, 33–37. [Google Scholar] [CrossRef]
- Llères, D.; Weibel, J.M.; Heissler, D.; Zuber, G.; Duportail, G.; Mély, Y. Dependence of the cellular internalization and transfection efficiency on the structure and physicochemical properties of cationic detergent/DNA/liposomes. J. Gene Med. 2004, 6, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Saito, S.; Takeda, N.; Takeoka, S. Plasmid DNA-encapsulating liposomes: Effect of a spacer between the cationic head group and hydrophobic moieties of the lipids on gene expression efficiency. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, S.; Holsaeter, A.M.; Skar, M.; Frantzen, C.B.; Brandl, M. Liposome size analysis by dynamic/static light scattering upon size exclusion-/field flow-fractionation. J. Nanosci. Nanotechnol. 2006, 6, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Zhu, T.F.; Szostak, J.W. Preparation of large monodisperse vesicles. PLoS ONE 2009, 4, e5009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Mori, A.; Huang, L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim. Biophys. Acta 1992, 1104, 95–101. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Ruozi, B.; Belletti, D.; Tombesi, A.; Tosi, G.; Bondioli, L.; Forni, F.; Vandelli, M.A. AFM, ESEM, TEM, and CLSM in liposomal characterization: A comparative study. Int. J. Nanomed. 2011, 6, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ruozi, B.; Tosi, G.; Forni, F.; Fresta, M.; Vandelli, M.A. Atomic force microscopy and photon correlation spectroscopy: Two techniques for rapid characterization of liposomes. Eur. J. Pharm. Sci. 2005, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Spyratou, E.; Mourelatou, E.A.; Makropoulou, M.; Demetzos, C. Atomic force microscopy: A tool to study the structure, dynamics and stability of liposomal drug delivery systems. Expert Opin. Drug Deliv. 2009, 6, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Spyratou, E.; Mourelatou, E.A.; Demetzos, C.; Makropoulou, M.; Serafetinides, A.A. Characterization of new drug delivery nanosystems using atomic force microscopy. In Proceedings of the SPIE 9447, 18th International School on Quantum Electronics: Laser Physics and Applications, Sozopol, Bulgaria, 8 January 2015. [Google Scholar] [CrossRef]
- Teschke, O.; De Souza, E.F. Liposome structure imaging by atomic force microscopy: Verification of improved liposome stability during adsorption of multiple aggregated vesicles. Langmuir 2002, 18, 6513–6520. [Google Scholar] [CrossRef]
- De Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [PubMed]
- Pick, U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch. Biochem. Biophys. 1981, 212, 186–194. [Google Scholar] [CrossRef]
- Aliño, S.F.; García, M.; Lejarreta, M.; Bobadilla, M.; Pérez-Yarza, G.; Unda, F.J. Trapping drug efficiency in liposomes produced by extrusion of freeze-thaw multilamellar vesicles. Biochem. Soc. Trans. 1989, 17, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Gjetting, T.; Andresen, T.L.; Christensen, C.L.; Cramer, F.; Poulsen, T.T.; Poulsen, H.S. A simple protocol for preparation of a liposomal vesicle with encapsulated plasmid DNA that mediate high accumulation and reporter gene activity in tumor tissue. Results Pharma Sci. 2011, 1, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.P.; Xu, X.; Burgess, D.J. Freeze-anneal-thaw cycling of unilamellar liposomes: Effect on encapsulation efficiency. Pharm. Res. 2014, 31, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.; Semple, S.C.; Klimuk, S.K.; Edwards, K.; Eisenhardt, M.L.; Leng, E.C.; Karlsson, G.; Yanko, D.; Cullis, P.R. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim. Biophys. Acta Biomembr. 2006, 1758, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Olson, F.; Hunt, C.A.; Szoka, F.C.; Vail, W.J.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta 1979, 557, 9–23. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Fuchs, B.; Süß, R.; Teuber, K.; Eibisch, M.; Schiller, J. Lipid analysis by thin-layer chromatography—A review of the current state. J. Chromatogr. A 2011, 1218, 2754–2774. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
 ), 37 °C (
), 37 °C (  ) and 45 °C (
) and 45 °C (  ) versus time. (Left panels): POPC formulation A0, B0 and C0; (Right panels): 60-PEPC formulation A60, B60 and C60. The red line shows the size threshold of 200 nm.
) versus time. (Left panels): POPC formulation A0, B0 and C0; (Right panels): 60-PEPC formulation A60, B60 and C60. The red line shows the size threshold of 200 nm.
 ), 37 °C (
), 37 °C (  ) and 45 °C (
) and 45 °C (  ) versus time. (Left panels): POPC formulation A0, B0 and C0; (Right panels): 60-PEPC formulation A60, B60 and C60. The red line shows the size threshold of 200 nm.
) versus time. (Left panels): POPC formulation A0, B0 and C0; (Right panels): 60-PEPC formulation A60, B60 and C60. The red line shows the size threshold of 200 nm.

| Formulation | Composition | Size (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|---|
| A0 | POPC | 149.1 ± 0.18 | 0.18 ± 0.01 | −6.03 ± 1.05 |
| A30 | 30-PEPC | 144.45 ± 0.78 | 0.11 ± 0.06 | −4.35 ± 0.95 |
| A60 | 60-PEPC | 117.40 ± 0.55 | 0.16 ± 0.01 | −2.98 ± 0.86 |
| B0 | POPC/CHOL 7:3 | 170.70 ± 2.19 | 0.31 ± 0.01 | −7.52 ± 1.23 |
| B30 | 30-PEPC/CHOL 7:3 | 167.80 ± 2.51 | 0.32 ± 0.01 | −7.22 ± 0.56 |
| B60 | 60-PEPC/CHOL 7:3 | 156.60 ± 1.03 | 0.17 ± 0.01 | −3.45 ± 0.97 |
| C0 | POPC/DPPC/CHOL/POPE/MTMAB 25:25:20:15:15 | 138.2 ± 0.95 | 0.09 ± 0.03 | +20.5 ± 1.45 |
| C30 | 30-PEPC/DPPC/CHOL/POPE/MTMAB 25:25:20:15:15 | 134.7 ± 1.85 | 0.31 ± 0.12 | +20.9 ± 0.98 |
| C60 | 60-PEPC/DPPC/CHOL/POPE/MTMAB 25:25:20:15:15 | 134.5 ± 0.28 | 0.13 ± 0.19 | +19.2 ± 1.11 |
| POPC | 60-PEPC | |
|---|---|---|
| Diameter (nm) | 145.00 ± 37.98 | 122.78 ± 13.15 |
| Height (nm) | 6.04 ± 1.22 | 9.28 ± 1.80 |
| Height/diameter ratio | 0.04 | 0.08 |
| Method | Procedure |
|---|---|
| A | Vortexing (10 min, 22 °C) |
| B | Vortexing (10 min, 40 °C) + 2 sonication cycles (5 min each) |
| C | Vortexing (10 min, 22 °C) + 2 freeze-annealing-thaw cycles |
| D | Vortexing (10 min, 22 °C) + 5 freeze-annealing-thaw cycles |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacometti, G.; Marini, M.; Papadopoulos, K.; Ferreri, C.; Chatgilialoglu, C. trans-Double Bond-Containing Liposomes as Potential Carriers for Drug Delivery. Molecules 2017, 22, 2082. https://doi.org/10.3390/molecules22122082
Giacometti G, Marini M, Papadopoulos K, Ferreri C, Chatgilialoglu C. trans-Double Bond-Containing Liposomes as Potential Carriers for Drug Delivery. Molecules. 2017; 22(12):2082. https://doi.org/10.3390/molecules22122082
Chicago/Turabian StyleGiacometti, Giorgia, Marina Marini, Kyriakos Papadopoulos, Carla Ferreri, and Chryssostomos Chatgilialoglu. 2017. "trans-Double Bond-Containing Liposomes as Potential Carriers for Drug Delivery" Molecules 22, no. 12: 2082. https://doi.org/10.3390/molecules22122082