HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo
Abstract
:1. Introduction
2. Results and Discussion
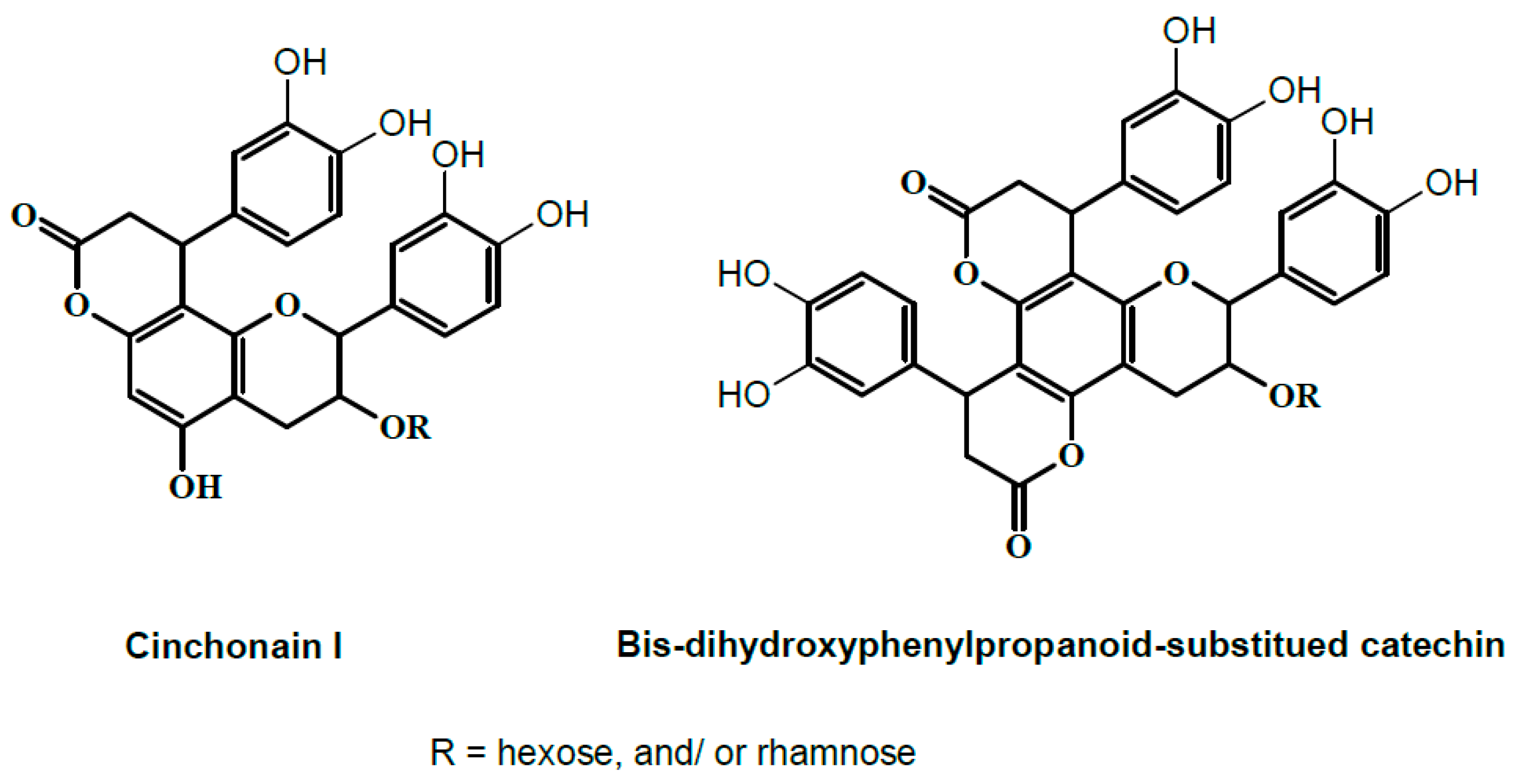
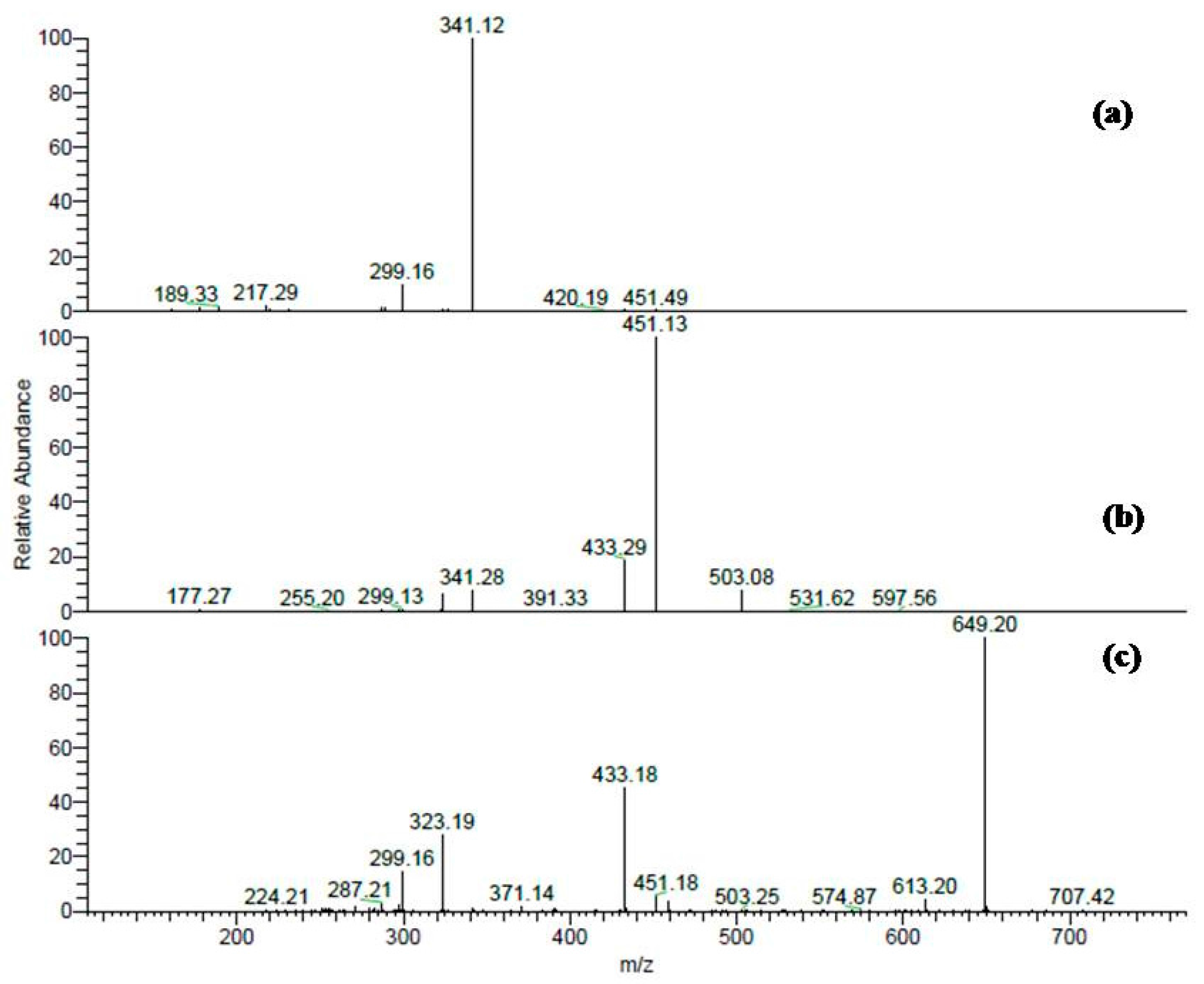
2.1. Phytochemicals in T. fischeri Bark
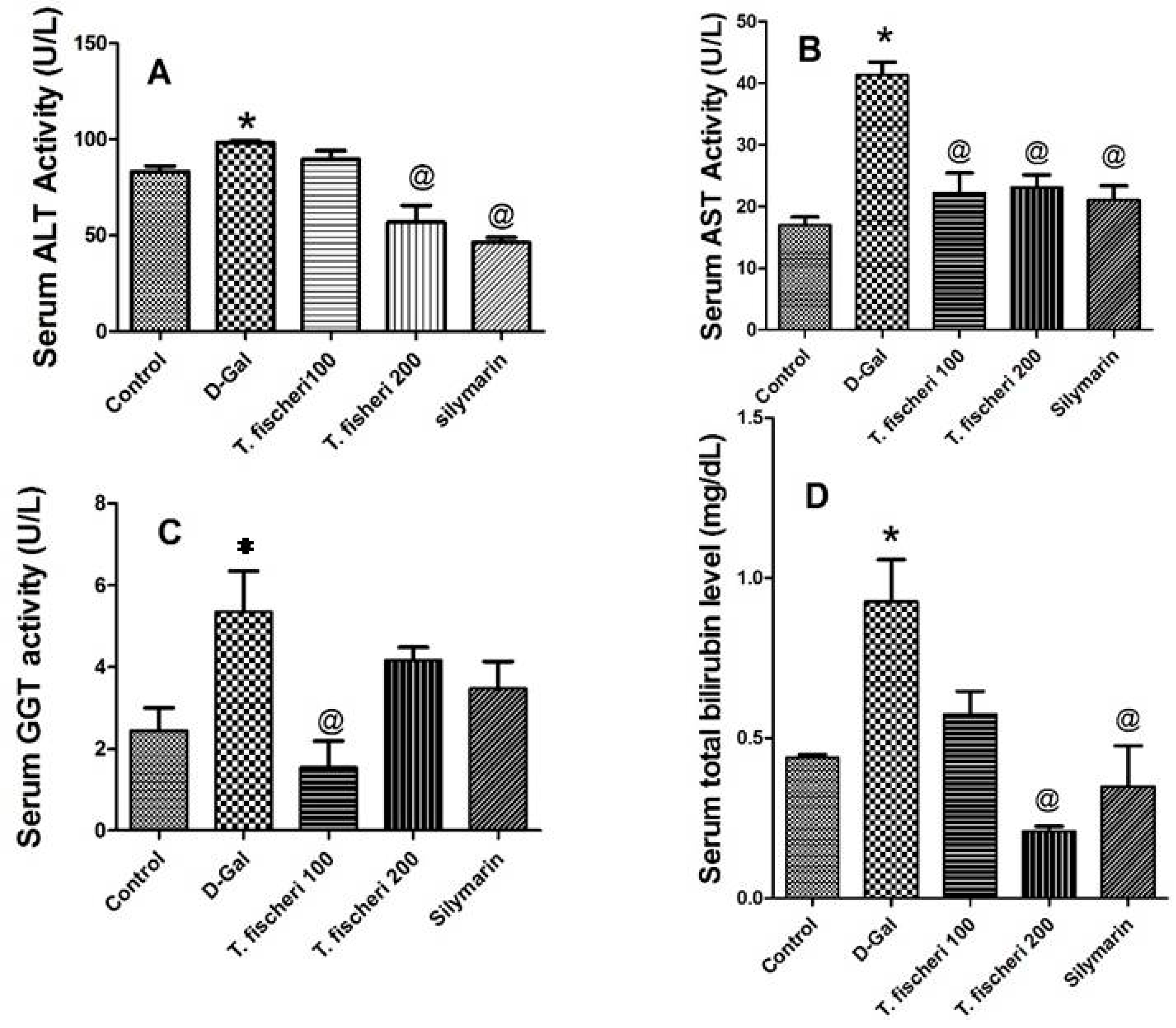
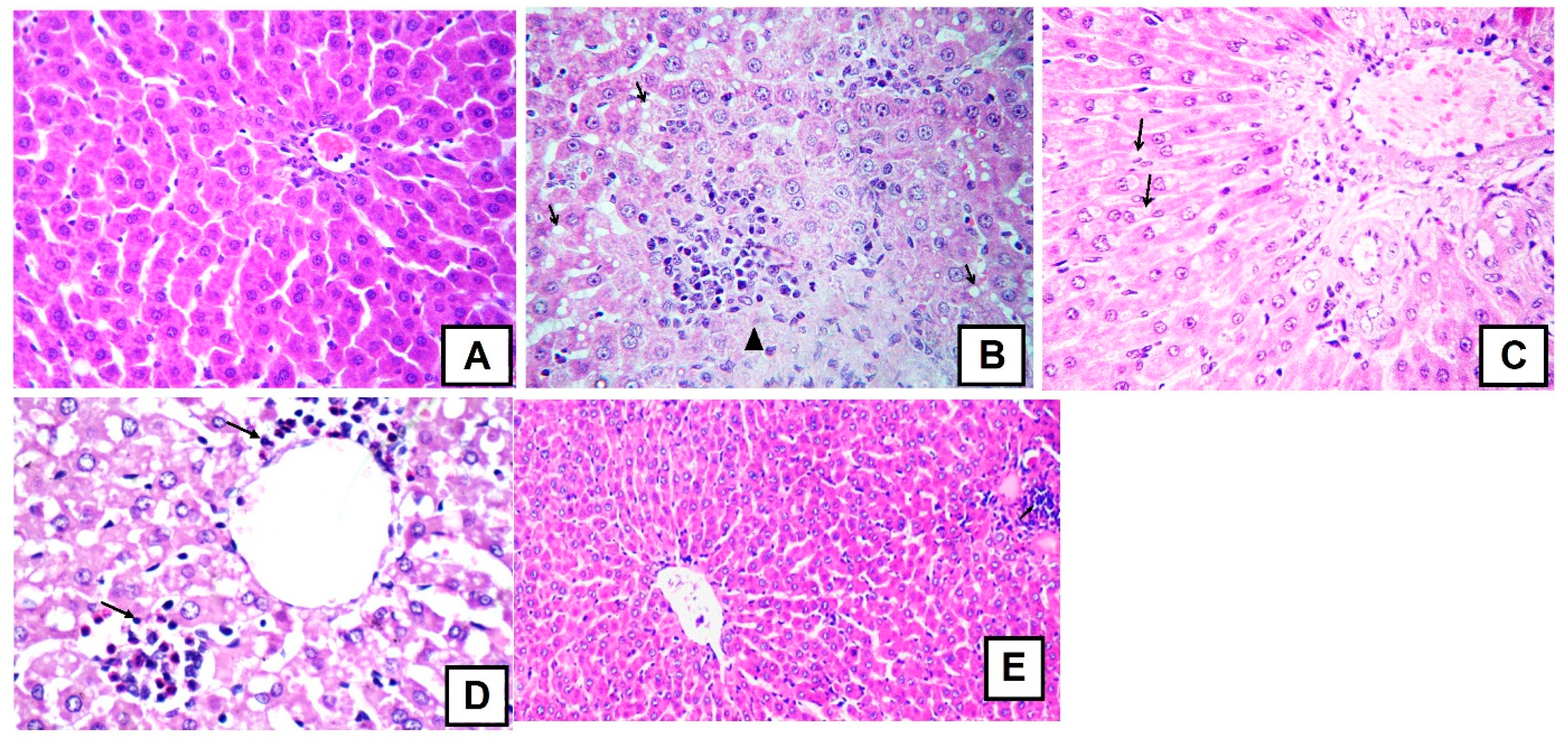
2.2. Antioxidant and Hepatoprotective Activities
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. HPLC-PDA-MS/MS
3.3. Antioxidant Activities
3.4. Hepatoprotective Experiments
3.4.1. Animals
3.4.2. Blood and Tissue Sampling
3.4.3. Biochemical Determinations
3.4.4. Histology Studies
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adly, A.A. Oxidative stress and disease: An updated review. Res. J. Immunol. 2010, 3, 129–145. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs and Poisons; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World, 2nd ed.; CABI: Wallingford, UK, 2017. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Yuan, C.-M.; Tang, G.-H.; Zhang, Y.; Wang, X.-Y.; Cao, M.-M.; Guo, F.; Li, Y.; Di, Y.-T.; Li, S.-L.; Hua, H.-M. Bioactive limonoid and triterpenoid constituents of Turraea pubescens. J. Nat. Prod. 2013, 76, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Mokoka, T.A.; Xolani, P.K.; Zimmermann, S.; Hata, Y.; Adams, M.; Kaiser, M.; Moodley, N.; Maharaj, V.; Koorbanally, N.A.; Hamburger, M. Antiprotozoal screening of 60 South African plants, and the identification of the antitrypanosomal germacranolides schkuhrin I and II. Planta Med. 2013, 79, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.O.; Saka, Y.A.; Fasinu, P.S.; Fadare, A.A.; Ajaiyeoba, E.O. Antimalarial and cytotoxic properties of Chukrasia tabularis A. Juss and Turraea vogelii Hook F. Ex. Benth. Parasitol. Res. 2016, 115, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Ashour, M.L.; Rubanza, C.D.; Wink, M. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother. Res. 2010, 24, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Neuwinger, H.D. African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology, Toxicology; CRC Press: Weinheim, Germany, 1996. [Google Scholar]
- Augustino, S.; Hall, J.B.; Makonda, F.B.; Ishengoma, R.C. Medicinal Resources of the Miombo woodlands of Urumwa, Tanzania: Plants and its uses. J. Med. Plants Res. 2011, 5, 6352–6372. [Google Scholar]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wink, M. Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan. PeerJ 2016, 4, e1879. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.Y.; Li, N.; Leung, E.L.H.; Zhou, H.; Yao, X.J.; Liu, L.; Wu, J.L. Rapid identification of new minor chemical constituents from Smilacis glabrae rhizoma by combined use of UHPLC-Q-TOF-MS, preparative HPLC and UHPLC-SPE-NMR-MS techniques. Phytochem. Anal. 2015, 26, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Resende, F.O.; Rodrigues-Filho, E.; Luftmann, H.; Petereit, F.; Mello, J.C. Phenylpropanoid substituted flavan-3-ols from Trichilia catigua and their in vitro antioxidative activity. J. Braz. Chem. Soc. 2011, 22, 2087–2093. [Google Scholar] [CrossRef]
- Tang, J.S.; Gao, H.; Wang, C.X.; Dai, Y.; Bao, L.; Ye, W.C.; Yao, X.S. Antioxidative phenylpropanoid-substituted epicatechin glycosides from Parabarium huaitingii. Planta Med. 2009, 75, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Kokke, B.P.; Leroux, M.R.; Candido, E.P.M.; Boelens, W.C.; de Jong, W.W. Caenorhabditis elegans small heat-shock proteins Hsp12.2 and Hsp12.3 form tetramers and have no chaperone-like activity. FEBS Lett. 1998, 433, 228–232. [Google Scholar] [CrossRef]
- Grünz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Spanier, B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012, 133, 1–10. [Google Scholar]
- Abbas, S.; Wink, M. Green tea extract induces the resistance of Caenorhabditis elegans against oxidative stress. Antioxidants 2014, 3, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.; Hasan, R.; Cheng, H.; El-Shazly, A.; Wink, M. Senna singueana: Antioxidant, hepatoprotective, antiapoptotic properties and phytochemical profiling of a methanol bark extract. Molecules 2017, 22, 1502. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Wink, M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009, 75, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 2010, 17, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.; El-Beshbishy, H.A.; El-Shazly, A.M.; Wink, M. Albizia harveyi: Phytochemical profiling, antioxidant, antidiabetic and hepatoprotective activities of the bark extract. Med. Chem. Res. 2017, 26, 3091–3105. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; El-Beshbishy, H.A.; El-Shazly, A.M.; Wink, M. Hepatoprotective and hypoglycemic effects of a tannin rich extract from Ximenia americana var. caffra root. Phytomedicine 2017, 33, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.-G.; Sobeh, M.; Wink, M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharm. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
Sample Availability: Samples of plant materials are available from the authors. |
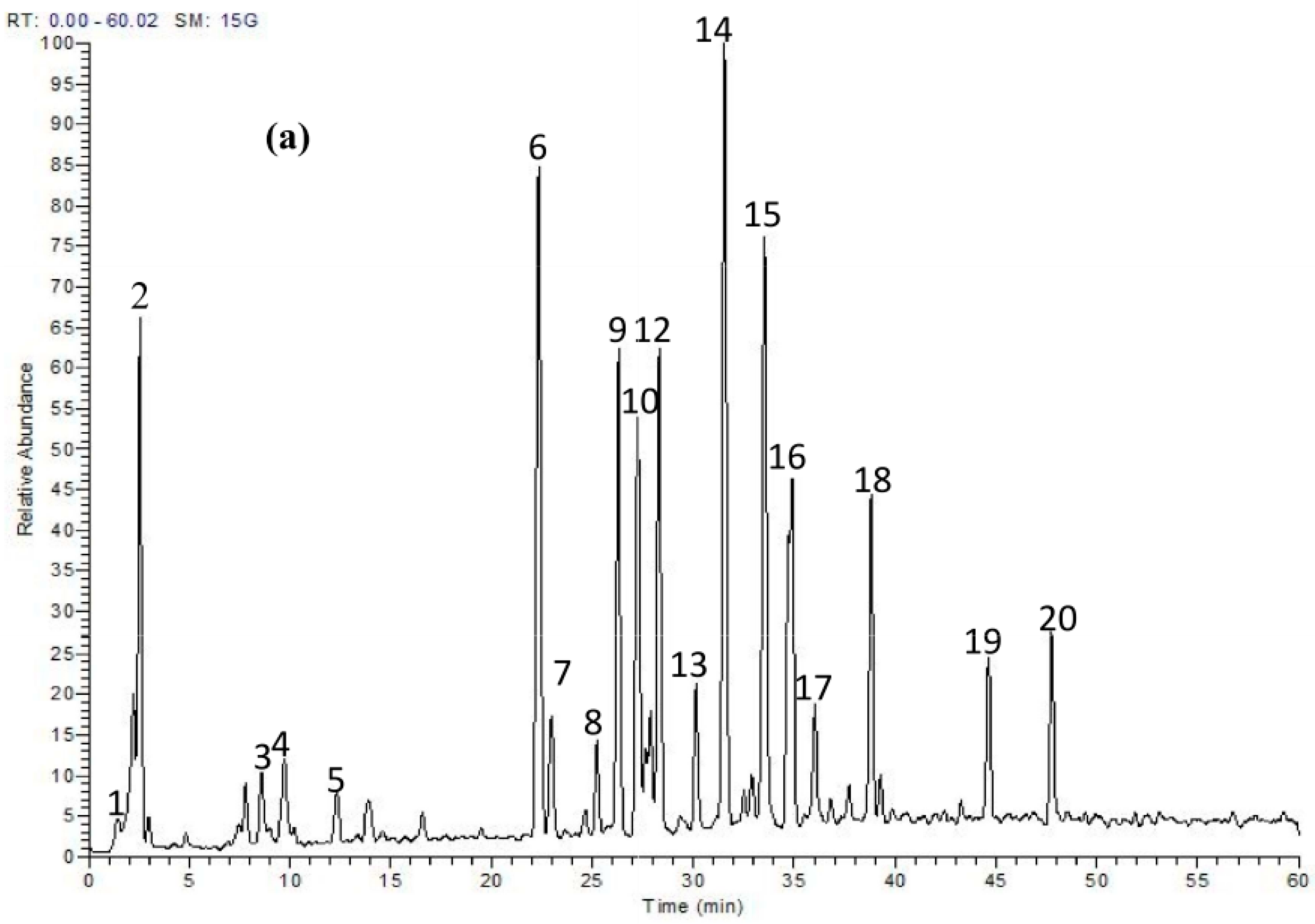
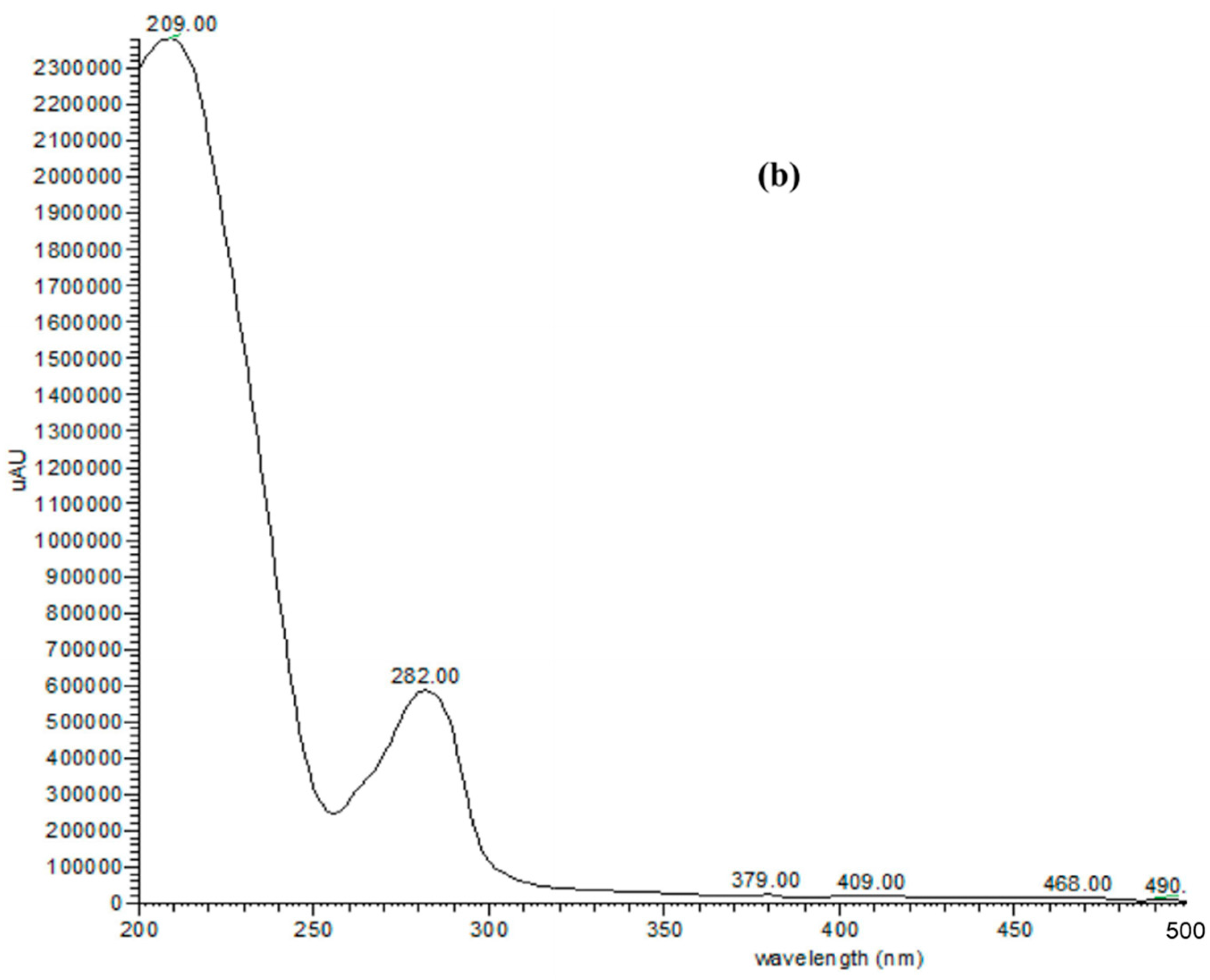

| No. | tR | [M − H]− | MS/MS Fragments | Tentatively Identified Compounds |
|---|---|---|---|---|
| 2 | 2.00 | 353 | 179, 191 | Chlorogenic acid |
| 1 | 1.65 | 447 | 315, 153 | Protocatechuic acid pentosyl-hexoside |
| 15 | 33.41 | 451 | 299, 341 | Cinchonain-I * |
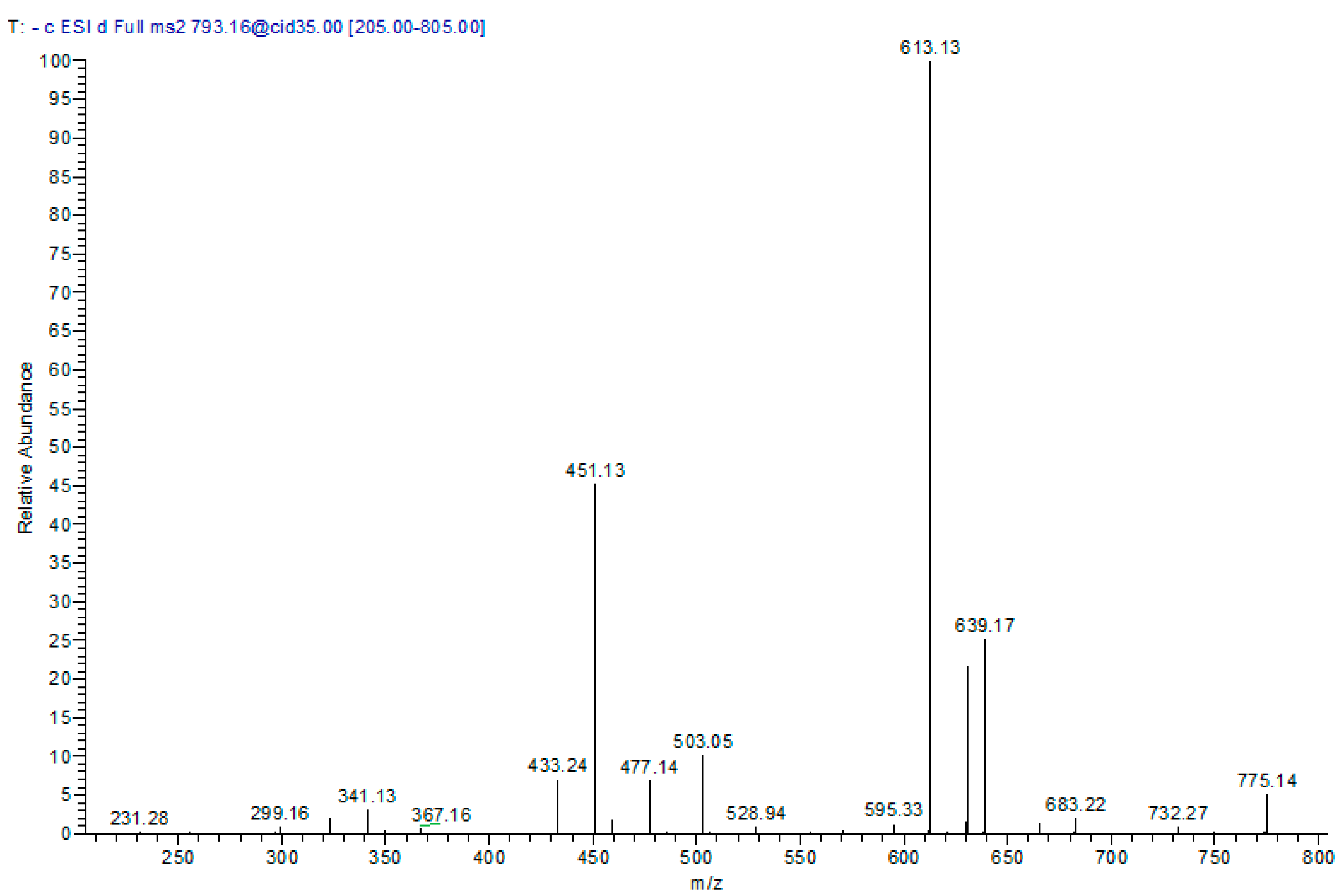
| 8 | 25.24 | 613 | 299, 341, 451, 595 | Cinchonain-I hexoside * |
| 9 | 26.27 | 613 | 299, 341, 451, 595 | Cinchonain-I hexoside * |
| 10 | 27.69 | 613 | 299, 341, 451, 595 | Cinchonain-I hexoside * |
| 6 | 22.39 | 759 | 299, 323, 433, 451, 613, 649 | Cinchonain-I rhamnosyl-hexoside * |
| 7 | 23.00 | 759 | 299, 323, 433, 451, 613, 649 | Cinchonain-I rhamnosyl-hexoside * |
| 11 | 27.18 | 759 | 299, 323, 433, 451, 613, 649 | Cinchonain-I rhamnosyl-hexoside * |
| 12 | 28.27 | 759 | 299, 323, 433, 451, 613, 649 | Cinchonain-I rhamnosyl-hexoside * |
| 13 | 30.11 | 759 | 299, 323, 433, 451, 613, 649 | Cinchonain-I rhamnosyl-hexoside * |
| 17 | 36.1 | 759 | 299, 323, 433, 451, 613, 649 | Cinchonain-I rhamnosyl-hexoside * |
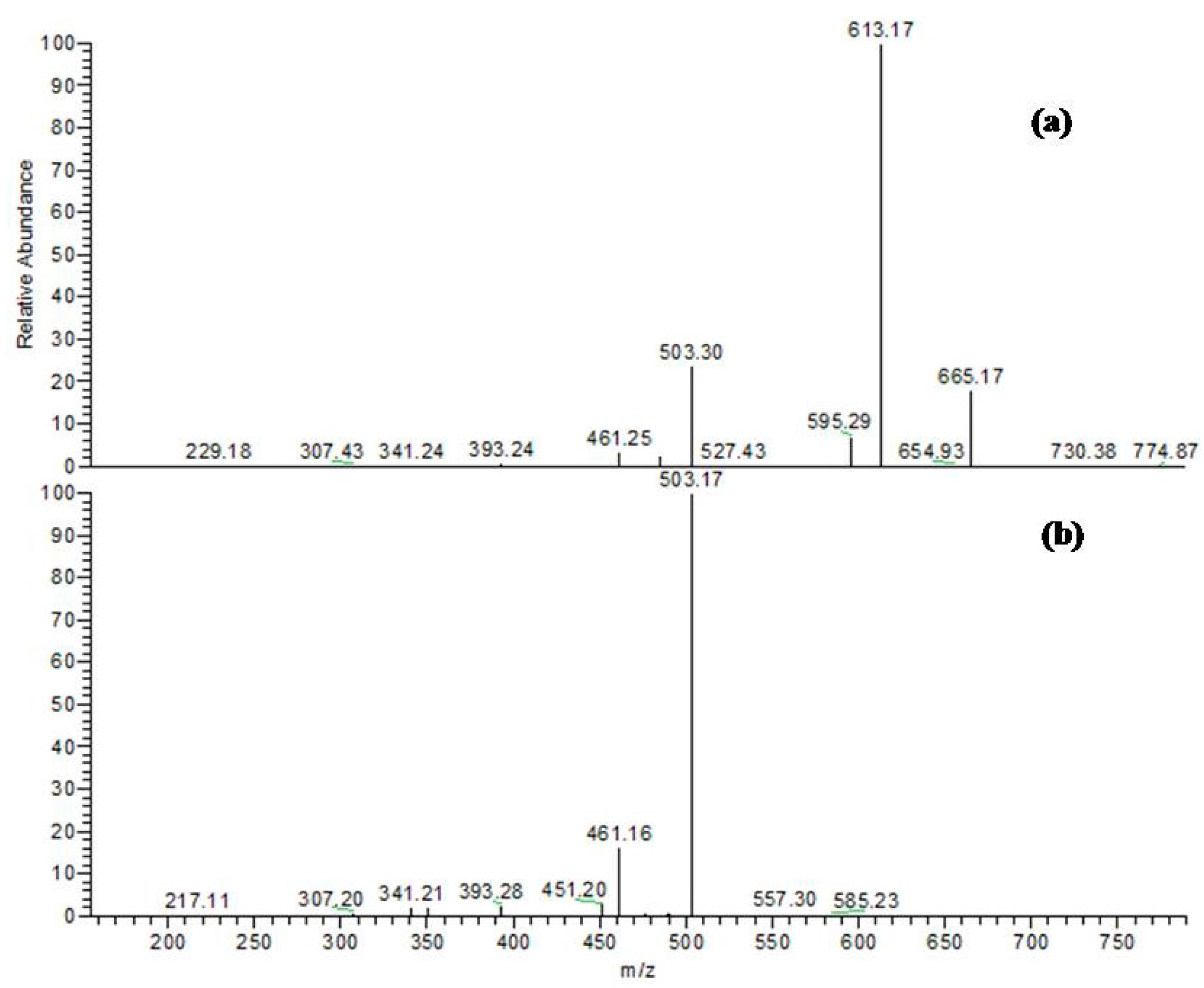
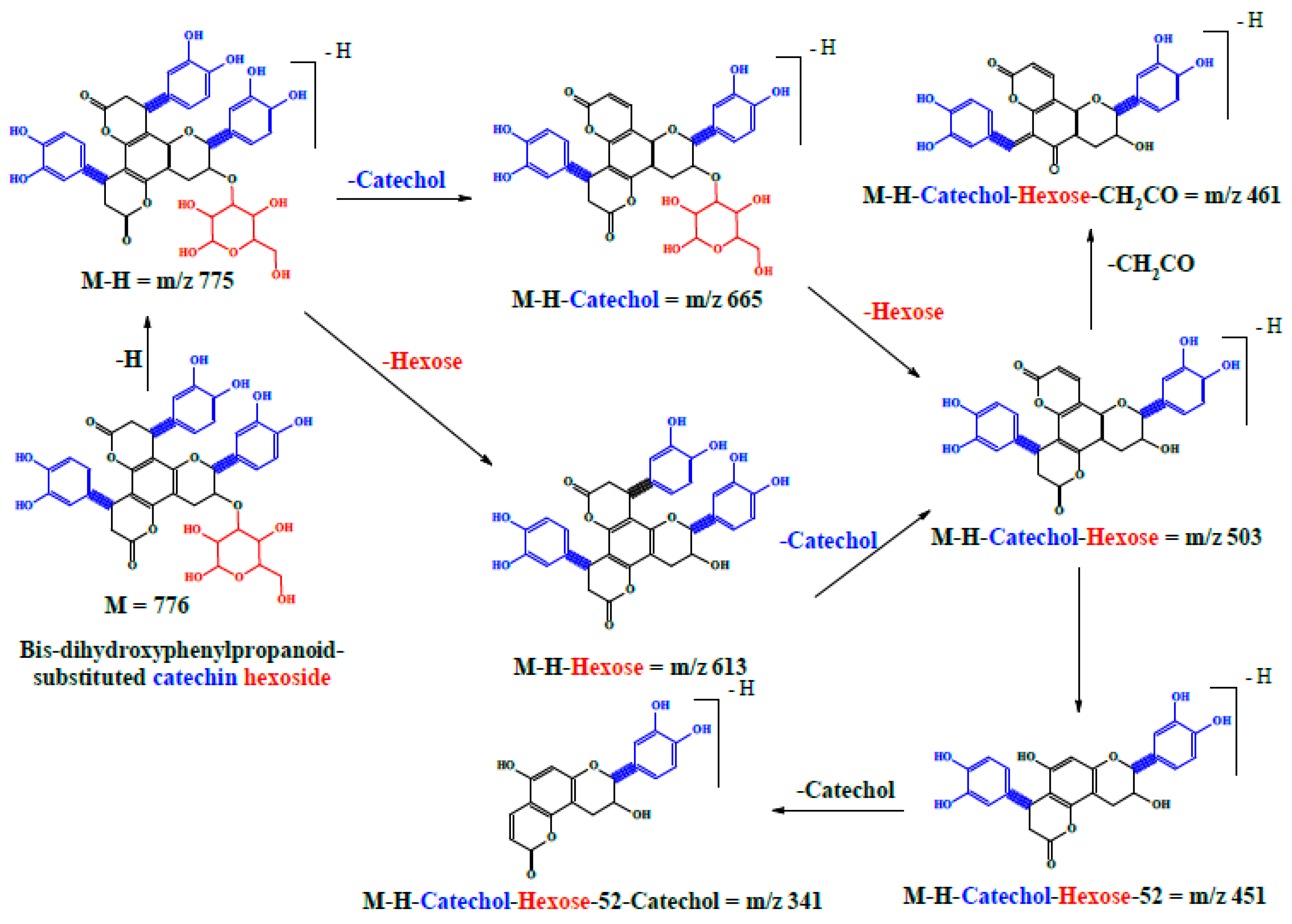
| 16 | 34.76 | 775 | 305, 393, 461, 503, 613, 665 | Bis-dihydroxyphenylpropanoid-substituted catechin hexoside ** |
| 20 | 47.74 | 775 | 305, 393, 461, 503, 613, 665 | Bis-dihydroxyphenylpropanoid-substituted catechin hexoside ** |
| 5 | 12.24 | 793 | 341, 299, 451, 503, 613, 639 | Cinchonain-I syringyl-hexoside * |
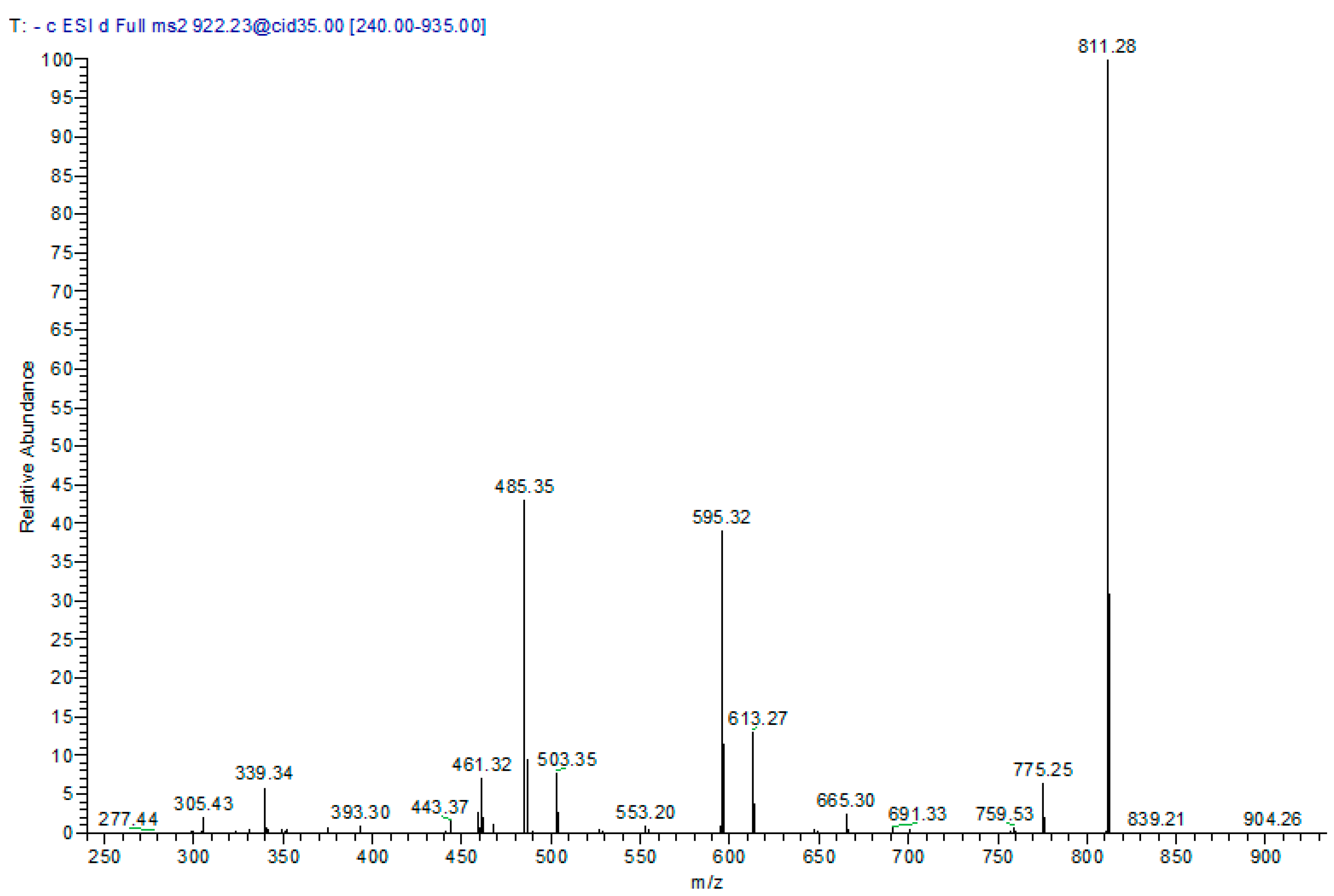
| 14 | 31.45 | 921 | 305, 461, 485, 595, 613, 811 | Bis-dihydroxyphenylpropanoid-substituted catechin rhamnosyl-hexoside ** |
| 18 | 38.77 | 921 | 305, 461, 485, 595, 613, 811 | Bis-dihydroxyphenylpropanoid-substituted catechin rhamnosyl-hexoside ** |
| 19 | 44.64 | 921 | 305, 461, 485, 595, 613, 811 | Bis-dihydroxyphenylpropanoid-substituted catechin rhamnosyl-hexoside ** |
| 3 | 8.54 | 939 | 289, 613, 759 | Bis-dihydroxyphenylpropanoid-substituted catechin syringyl-rhamnoside ** |
| 4 | 9.72 | 939 | 289, 433, 613, 759 | Bis-dihydroxyphenylpropanoid-substituted catechin syringyl-rhamnoside ** |
| Sample | Bark Extract | AscorbicAcid |
|---|---|---|
| DPPH (EC50, µg/mL) | 5.12 | 3.31 |
| FRAP (mM FeSO4/mg extract) | 18.32 | 22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobeh, M.; Mahmoud, M.F.; Sabry, O.M.; Adel, R.; Dmirieh, M.; El-Shazly, A.M.; Wink, M. HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo. Molecules 2017, 22, 2089. https://doi.org/10.3390/molecules22122089
Sobeh M, Mahmoud MF, Sabry OM, Adel R, Dmirieh M, El-Shazly AM, Wink M. HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo. Molecules. 2017; 22(12):2089. https://doi.org/10.3390/molecules22122089
Chicago/Turabian StyleSobeh, Mansour, Mona F. Mahmoud, Omar M. Sabry, Rasha Adel, Malak Dmirieh, Assem M. El-Shazly, and Michael Wink. 2017. "HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo" Molecules 22, no. 12: 2089. https://doi.org/10.3390/molecules22122089






