The Antitumor Constituents from Hedyotis Diffusa Willd
Abstract
:1. Introduction
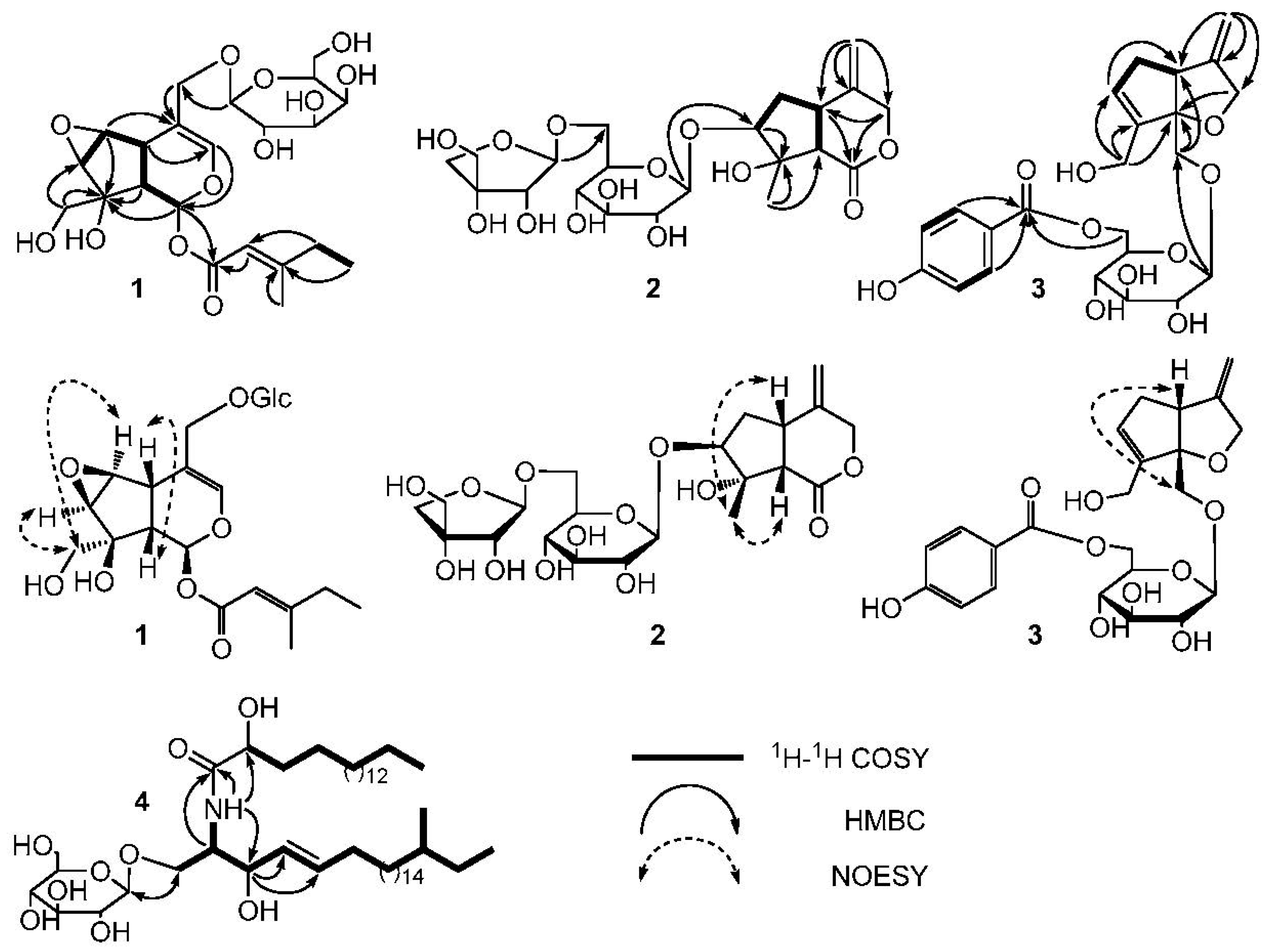
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of 1–3
3.5. Methanolysis of 4
3.6. Cytotoxicity Assay of Compounds 1–10
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, Q.Y.; Lin, J.M.; Wei, L.H.; Zhang, L.; Wang, L.L.; Zhan, Y.Z.; Zeng, J.W.; Xu, W.; Shen, A.L.; Hong, Z.F.; et al. Hedyotis diffusa Willd Inhibits Colorectal Cancer Growth in Vivo via Inhibition of STAT3 Signaling Pathway. Int. J. Mol. Sci. 2012, 13, 6117–6128. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Meng, Q.X. Chemical and preclinical studies on Hedyotis diffusa with anticancer potential. J. Asian Nat. Prod. Res. 2013, 15, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. Preparation of Graphene-Multi-Walled Carbon Nanotube Composite for Quantitive Determination of 2-hydroxy-3- Methylanthraquinone in Hedyotis diffusa. Int. J. Electrochem. Sci. 2017, 12, 629–638. [Google Scholar]
- Chao, T.H.; Fu, P.K.; Chang, C.H.; Chang, S.N.; Chiahung, M.F.; Lin, C.H. Prescription patterns of Chinese herbal products for post-surgery colon cancer patients in Taiwan. J. Ethnopharmacol. 2014, 155, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Chen, H.Y.; Yang, S.H.; Lin, Y.H.; Chiu, J.H.; Lin, Y.H.; Chen, J.L. Hedyotis diffusa Combined with Scutellaria barbata Are the Core Treatment of Chinese Herbal Medicine Used for Breast Cancer Patients: A Population-Based Study. Evid. Based Complement. Altern. Med. 2014, 2014, 202378. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; He, J.; Tong, X.; Tang, L.; Liu, M. The Hedyotis diffusa willd. (rubiaceae): A review on phytochemistry, pharmacology, quality control and pharmacokinetics. Molecules 2016, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Liu, M.H.; Zhang, X.L.; He, J.Y. Chemical profiles and protective effect of Hedyotis diffusa willd in lipopolysaccharide-induced renal inflammation mice. Int. J. Mol. Sci. 2015, 16, 27252–27269. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Schmitz, O.J. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass QTOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal. Bioanal. Chem. 2015, 407, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.Q.; Cheng, C.S.; Liu, Z.Z.; Ouyang, Y.; Lin, J.R.; Zhang, Z.F.; Liu, Z.Q.; Zhou, H. Comparison of identification and medicinal progress of Hedyotis diffusa and H. corymbosa. Chin. Tradit. Herb. Drugs 2017, 48, 4328–4338. [Google Scholar]
- Chen, Y.; Lin, Y.; Yachan, L.I.; Candong, L.I. Total flavonoids of Hedyotis diffusa willd inhibit inflammatory responses in lps-activated macrophages via suppression of the nf-κb and mapk signaling pathways. Exp. Ther. Med. 2016, 11, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Bai, Y.; Huo, Z. The protective effect of Hedyotis diffusa on collagen induced arthritis rats. Int. J. Clin. Exp. Med. 2016, 9, 12880–12887. [Google Scholar]
- Gao, X.; Li, C.; Tang, Y.L.; Zhang, H. Chan, S.W. Effect of Hedyotis diffusa water extract on protecting human hepatocyte cells (LO) from HO-induced cytotoxicity. Pharm. Biol. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Lin, J.P.; Hsiao, Y.T.; Chou, G.L.; Tsai, Y.H.; Chiang, S.Y.; Lin, J.G.; Chung, J.G. Ethanol Extract of Hedyotis diffusa Willd Affects Immune Responses in Normal Balb/c Mice In Vivo. In Vivo 2015, 29, 453–460. [Google Scholar] [PubMed]
- Hu, E.; Wang, D.; Chen, J.; Tao, X. Novel cyclotides from Hedyotis diffusa induce apoptosis and inhibit proliferation and migration of prostate cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 4059–4065. [Google Scholar] [PubMed]
- Zhang, Y.; Xie, R.F.; Xiao, Q.G.; Li, R.; Shen, X.L.; Zhu, X.G. Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J. Ethnopharmacol. 2014, 158, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Liu, Y.J.; Way, T.D. Synergistic inhibition of leukemia wehi-3 cell growth by arsenic trioxide and Hedyotis diffusa willd extract in vitro and in vivo. Exp. Ther. Med. 2017, 13, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, B.; Li, J.; Ye, B.; Lin, S.; Qian, W.; Shan, L.; Efferthd, T. Total coumarins of Hedyotis diffusa, induces apoptosis of myelodysplastic syndrome skm-1 cells by activation of caspases and inhibition of pi3k/akt pathway proteins. J. Ethnopharmacol. 2017, 196, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Qi, B.; Jiang, G.; Liu, J.; Zhang, P.; Ma, Y.; Li, W. The anti-tumor effect and bioactive phytochemicals of Hedyotis diffusa willd on ovarian cancer cells. J. Ethnopharmacol. 2016, 192, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Chang, L.; Tang, Y.L.; Zhang, H.; Chan, S.W. Effect of Hedyotis diffusa water extract on protecting human hepatocyte cells (lo2) from H2O2-induced cytotoxicity. Pharm. Biol. 2016, 54, 1148–1155. [Google Scholar]
- Sun, G.; Wei, L.; Feng, J.; Lin, J.; Peng, J. Inhibitory effects of Hedyotis diffusa willd on colorectal cancer stem cells. Oncol. Lett. 2016, 11, 3875–3881. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Ahmedin, J.; Xue, Q.Y.; Jie, H. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Li, S.Y.; Li, W.J.; Guo, J.M.; Tian, K.; Hu, Q.F.; Huang, X.Z. Phenolic glycosides from Ficus tikoua and their cytotoxic activities. Carbohydr. Res. 2013, 382C, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, T.; Liu, X.H.; Shen, Y.H.; Li, H.L.; Shan, L.; Liu, R.H.; Xu, X.K.; Zhang, W.D.; Wang, H. Iridoids and lignans from Valeriana jatamansi. J. Nat. Prod. 2010, 73, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Di, L.; Gao, W.C.; Wang, K.J.; Zu, L.B. Cytotoxic iridoids from the roots of Patrinia scabra. J. Nat. Prod. 2012, 75, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Kawashima, K.; Sakagami, M.; Shimomura, M.; Ohashi, K.; Kitagawa, I. Sphingolipids and glycerolipids. Ι. Chemical structures and ionophoretic activities of soyacerebrosides Ι and ΙΙ form soybean. Chem. Pharm. Bull. 1990, 38, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Satoshi, K.; Kazufumi, N.; Masanori, I.; Ryuichi, H. Isolation and Structure Determination of Six Glucocerebrosides from the Starfish Luidia maculate. Chem. Pharm. Bull. 2002, 50, 1091–1096. [Google Scholar]
- Koji, Y.; Noriko, W.; Hiroyuki, O.; Rei, M.; Ryuichi, I.; Masanori, I.; Ryuichi, H. Constituents of Holothuroidea, Isolation of Ante-iso Type Regio isomer on Long Chain Base Moiety of Glucocerebroside from the Sea Cucumber Holothuria leucospilota. Chem. Pharm. Bull. 2005, 53, 788–791. [Google Scholar]
- Dinda, B.; Debnath, S.; Banik, R. Naturally occurring iridoids and secoiridoids. An updated review, part 4. Chem. Pharm. Bull. 2011, 59, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Yu, L.L.; Huang, R.; Lv, Y.P.; Gui, S.H. 11-methoxyviburtinal, a new iridoid from Valeriana jatamansi. Arch. Pharmacal. Res. 2005, 28, 1161–1163. [Google Scholar] [CrossRef]
- Iwagawa, T.; Yaguchi, S.; Hase, T. Iridoid glucosides from viburnum suspensum. Phytochemistry 1990, 29, 310–312. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Gong, Z.F.; Duan, X.Y.; Liu, Y.W. Two new terpenoids from Valeriana officinalis. Chin. J. Nat. Med. 2009, 7, 270–273. [Google Scholar] [CrossRef]
- Nishiya, K.; Kimura, T.; Takeya, K.; Itokawa, H. Sesquiterpenoids and iridoid glycosides from Valeriana fauriei. Phytochemistry 1992, 31, 3511–3514. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem. Pharm. Bull. 2007, 38, 689–728. [Google Scholar] [CrossRef]
- Teinkela, J.E.; Noundou, X.S.; Nguemfo, E.L.; Meyer, F.; Djoukoue, A.; Van Antwerpen, P.; Ngouela, S.; Tsamo, E.; Mpondo, E.A.; Vardamides, J.C.; et al. Identification of compounds with anti-proliferative activity from the wood of Ficus elastica Roxb. ex Hornem. aerial roots. Fitoterapia 2016, 112, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Murshid, S.S.A.; Badr, J.M.; Youssef, D.T.A. Penicillosides A and B: New cerebrosides from the marine-derived fungus Penicillium, species. Rev. Bras. Farmacogn. 2016, 26, 29–33. [Google Scholar] [CrossRef]
- Tian, X.R.; Tang, H.F.; Feng, J.T.; Li, Y.S.; Lin, H.W.; Fan, X.P.; Zhang, X. Neritinaceramides A–E, New Ceramides from the Marine Bryozoan Bugula neritina inhabiting South China Sea and their cytotoxicity. Mar. Drugs 2014, 12, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Cateni, F.; Zilic, J.; Zacchigna, M.; Procida, G. Cerebrosides with antiproliferative activity from Euphorbia peplis L. Fitoterapia 2010, 81, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Li, J.; Li, B.; Zhang, N.; Liu, H. Separation and identification of four new compounds with antibacterial activity from Portulaca oleraceal. Molecules 2015, 20, 16375–16387. [Google Scholar] [CrossRef] [PubMed]
- Nguen, T.H.; Pham, H.V.; Pham, N.K.; Quach, N.D.; Pudhom, K.; Hansen, P.E.; Phung Nguyen, K.P. Chemical constituents from Sonneratia ovata backer and their in vitro cytotoxicity and acetylcholinesterase inhibitory activities. Bioorg. Med. Chem. Lett. 2015, 25, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Shecaoiridoidside A–C and shecaocerenoside A are available from the authors. |

| 1 a | 2 a | 3 a | 4 b | ||
|---|---|---|---|---|---|
| H | δH (J, Hz) | δH (J, Hz) | δH (J, Hz) | H | δH (J, Hz) |
| 1 | 6.41, d (2.0) | 3.96, d (10.4); 3.76, d (10.4) | NH | 8.35, d, (8.4) | |
| 3 | 6.40, brs | 5.10, d (11.0) 4.44, d (11.6) | 4.37, d (12.6); 4.18, d (12.6) | 1 | 4.22, m 4.72, m |
| 5 | 3.09, brd (8.5) | 3.34, m | 3.25, m | 2 | 4.78, m |
| 6 | 4.04, d (2.5) | 2.34, dd (8.4, 13.4); 2.19, m | 2.74, m; 2.26, brd (16.4) | 3 | 4.77, m |
| 7 | 3.36, d (2.5) | 3.87, m | 5.78, brs | 4 | 5.86, m |
| 9 | 2.05, m | 3.08, d (10.7) | 5 | 5.98, m | |
| 10 | 3.69, d (2.8) | 1.59, s | 4.11, brd (10.0) | 6 | 2.06, m |
| 11 | 4.21, d (11.6); 4.35, d (11.6) | 5.08, 5.11, s | 4.92, 4.91, d (2.0) | 7–22 | 1.16–1.42, brs |
| 1′ | 4.41, d (7.8) | 4.72, d (7.8) | 23 | 0.88, d (6.4) | |
| 2′ | 5.62, s | 3.16, t (8.2) | 3.27, m | 24 | 0.86, t (6.4) |
| 3′ | 3.30, m | 3.43, m | 2′ | 4.61, m | |
| 4′ | 2.16, m | 3.29, m | 3.40, m | 3′ | 1.85, m |
| 5′ | 0.89, t (7.4) | 3.34, m | 3.62, m | 4′ | 1.73, m; 1.16–1.42, brs |
| 6′ | 2.15, s | 3.59, 3.95, m | 4.60, brd, (11.6); 4.42, dd, (11.8, 4.8) | 5′–17′ | 1.16–1.42, brs |
| 1”” | 4.72, d (8.1) | 5.01, d (1.7) | 18′ | 0.88, t (6.4) | |
| 2” | 3.35, m | 3.87, m | 7.88, d (8.8) | 1” | 4.90, d, (7.6) |
| 3” | 4.05, m | 6.81, d (8.8) | 2” | 4.02, m | |
| 4” | 3.49, m | 3.95, 3.75, m | 3” | 4.22, m | |
| 5” | 3.59 (1H, m) | 3.58, s | 6.81, d (8.8) | 4” | 4.22, m |
| 6” | 3.67, m; 3.86, dd (1.5, 11.5) | 7.88, d (8.8) | 5” | 3.88, m | |
| 6” | 4.36, 4.50, m | ||||
| 1 a | 2 a | 3 a | 4 b | ||||
|---|---|---|---|---|---|---|---|
| C | δC | C | δC | C | δC | C | δC |
| 1 | 90.8, CH | 1 | 175.2, C | 1 | 72.8, CH2 | 1 | 70.2, CH2 |
| 3 | 142.4, CH | 3 | 71.5, CH2 | 3 | 72.8, CH2 | 2 | 54.5, CH |
| 4 | 109.8, C | 4 | 144.5, C | 4 | 156.2, C | 3 | 72.3, CH |
| 5 | 35.4, CH | 5 | 41.2, CH | 5 | 49.8, CH | 4 | 131.6, CH |
| 6 | 59.9, CH | 6 | 40.0, CH2 | 6 | 39.2, CH2 | 5 | 132.7, CH |
| 7 | 60.3, CH | 7 | 90.1, CH | 7 | 131.4, CH | 6 | 34.2, CH2 |
| 8 | 80.2, C | 8 | 86.1, C | 8 | 144.4, C | 7-20 | 29.5–30.5, CH2 |
| 9 | 43.6, CH | 9 | 54.2, CH | 9 | 99.8, C | 21 | 35.7, CH |
| 10 | 67.2, CH2 | 10 | 22.5, CH3 | 10 | 59.3, CH2 | 22 | 30.5, CH2 |
| 11 | 69.8, CH2 | 11 | 113.8, CH2 | 11 | 105.4, CH2 | 23 | 19.6, CH3 |
| 1′ | 165.8, C | 1′ | 99.9, CH | 1′ | 103.9, CH | 24 | 11.8, CH3 |
| 2′ | 114.6, CH | 2′ | 75.5, CH | 2′ | 74.8, CH | 1′ | 175.6, C |
| 3′ | 162.1, C | 3′ | 78.7, CH | 3′ | 77.7, CH | 2′ | 72.5, CH |
| 4′ | 33.8, CH2 | 4′ | 72.3, CH | 4′ | 72.3, CH | 3′ | 35.8, CH2 |
| 5′ | 11.7, CH3 | 5′ | 78.3, CH | 5′ | 76.0, CH | 4′ | 26.2, CH2 |
| 6′ | 19.0, CH3 | 6′ | 68.3, CH2 | 6′ | 65.2, CH2 | 5′-15′ | 29.5–30.5, CH2 |
| 1” | 100.4, CH | 1” | 111.5, CH | 1” | 122.4, C | 16′ | 32.2, CH2 |
| 2” | 72.6, CH | 2” | 76.2, CH | 2” | 132.9, CH | 17′ | 22.8, CH2 |
| 3” | 73.2, CH | 3” | 80.8, C | 3” | 116.6, CH | 18′ | 14.2, CH3 |
| 4” | 69.2, CH | 4” | 75.4, CH2 | 4” | 164.2, C | 1” | 105.6, CH |
| 5” | 75.6, CH | 5” | 65.8, CH2 | 5” | 116.6, CH | 2” | 75.2, CH |
| 6” | 63.4, CH2 | 6” | 132.9, CH | 3” | 78.6, CH | ||
| 7” | 167.8, C | 4” | 71.5, CH | ||||
| 5” | 78.7, CH | ||||||
| 6” | 62.6, CH2 | ||||||
| Compounds | HL-60 | Hela | HCT15 | A459 | HepG2 | PC-3 | CNE-2 | BGC-823 |
|---|---|---|---|---|---|---|---|---|
| 1 | >100.0 | >100.0 | 87.6 ± 1.2 | 77.7 ± 1.6 | 37.6 ± 1.4 | >100.0 | >100.0 | >100.0 |
| 2 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 |
| 3 | 17.1 ± 0.7 | 62.2 ± 0.5 | 9.6 ± 0.8 | 14.8 ± 0.9 | 11.4 ± 1.6 | 26.2 ± 1.3 | 21.5 ± 0.6 | 13.4 ± 1.1 |
| 4 | 74.8 ± 1.3 | 89.3 ± 1.8 | 37.3 ± 1.5 | 33.6 ± 1.1 | 49.5 ± 1.4 | 64.0 ± 0.9 | 55.2 ± 1.1 | 44.1 ± 1.7 |
| 5 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 |
| 6 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 |
| 7 | >100.0 | >100.0 | 71.3 ± 1.2 | 50.4 ± 1.1 | >100.0 | 34.2 ± 1.3 | >100.0 | >100.0 |
| 8 | >100.0 | >100.0 | 89.8 ± 1.2 | 91.3 ± 0.7 | >100.0 | >100.0 | >100.0 | >100.0 |
| 9 | >100.0 | >100.0 | 96.1 ± 1.6 | 78.3 ± 0.8 | 97.9 ± 1.4 | >100.0 | >100.0 | >100.0 |
| 10 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 | >100.0 |
| 5-Fluorouracil | 7.5 ± 0.6 | 10.4 ± 0.4 | 4.7 ± 0.4 | 14.7 ± 1.1 | 22.8 ± 1.4 | 13.2 ± 0.7 | 11.6 ± 0.8 | 17.8 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhou, X.; Wang, Y.; Wei, D.; Deng, C.; Xu, X.; Xin, P.; Sun, S. The Antitumor Constituents from Hedyotis Diffusa Willd. Molecules 2017, 22, 2101. https://doi.org/10.3390/molecules22122101
Wang C, Zhou X, Wang Y, Wei D, Deng C, Xu X, Xin P, Sun S. The Antitumor Constituents from Hedyotis Diffusa Willd. Molecules. 2017; 22(12):2101. https://doi.org/10.3390/molecules22122101
Chicago/Turabian StyleWang, Changfu, Xuegang Zhou, Youzhi Wang, Donghua Wei, Chengjie Deng, Xiaoyun Xu, Ping Xin, and Shiqin Sun. 2017. "The Antitumor Constituents from Hedyotis Diffusa Willd" Molecules 22, no. 12: 2101. https://doi.org/10.3390/molecules22122101




